Experiments
Tensile and four-point bend fracture experiments were conducted to measure modulus and interfacial fracture energy, respectively. Angle-resolved x-ray photoelectron spectroscopy (XPS) was used to quantify the atomic makeup of the film surface.
Crosslinker Effects on Adhesion
The modulus of a polymer is a measure of the stiffness of the material. Therefore, a simple method of reducing the modulus is to reduce the amount of crosslinker material in the formulation. For this experiment, we tested formulations with varying crosslinker concentrations - 5-20 wt% ethylene glycol diacrylate (EGDA) - to see how this affected the modulus and interfacial fracture energy.
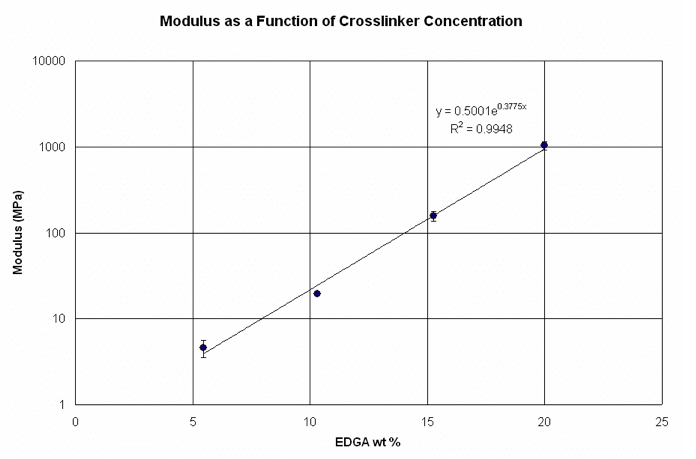
Figure 4. Tensile experimental results showing modulus as a function of EGDA concentration.
Clearly, the EGDA concentration has a significant impact on the film modulus. The relationship between the two variables appears to be exponential, which indicates that extremely low modulus values can be obtained by simply adjusting the crosslinker concentration. On the other hand, at very low crosslinker concentrations, the film becomes too soft and the imprinted patterns have trouble maintaining their shape. This effect may subsequently lead to poor pattern transfer. Therefore, imprints of the various formulations were generated using a repeating brick pattern template with 200 nm tall features to determine the minimum crosslinker concentration that could be used without sacrificing mechanical integrity. The sidewall angles of the imprinted patterns were then measured and compared as shown in Figure 5.
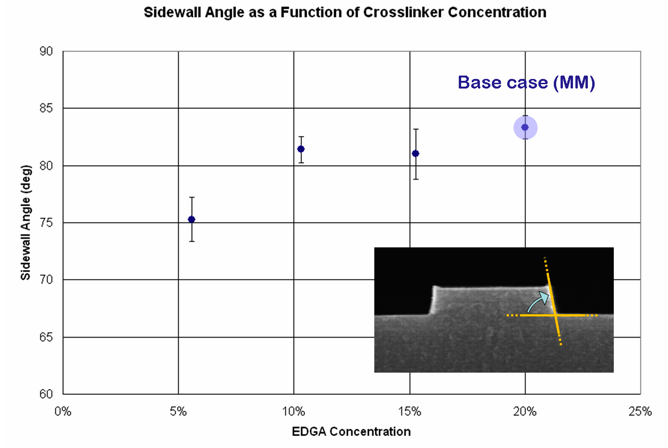
Figure 5. Sidewall angle as a function of EGDA concentration.
The sidewall angle of the imprinted patterns begins to decrease significantly when the crosslinker concentration drops below 10%. Thus, the minimum EGDA concentration was set at 10 %, which puts a limit on the extent the film modulus can be adjusted.
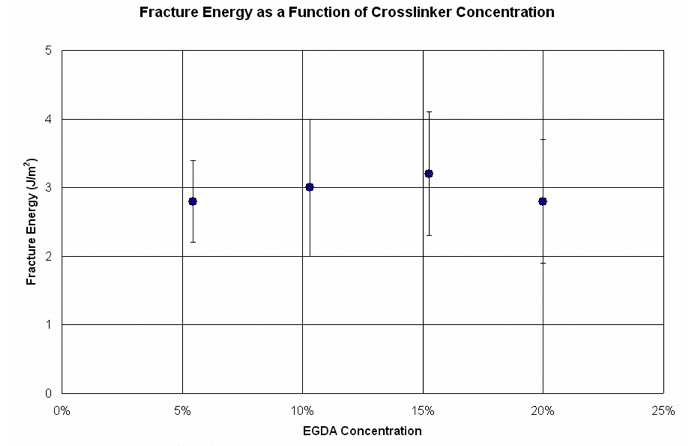
Figure 6. Fracture energy as a function of EGDA concentration.
On the other hand, changing the crosslinker concentration has no significant effect on the interfacial fracture energy. In order to minimize the overall separation force, it is necessary to decrease both modulus and interfacial fracture energy. Therefore, adjusting the crosslinker concentration does not appear to be a viable solution if a FSAM-treated template is used.
Surfactant Effects on Adhesion
Adding fluorinated surfactants to the etch barrier formulation may help decrease the separation force, while protecting the FSAM from physical or chemical attack. Fluorinated surfactant compounds selectively migrate to specific interfaces based on the free energy of the system, and the formation of a thin fluorocarbon layer between the template and polymer imprint is favorable for separation: this layer provides ideal separation properties such as low surface energy and low modulus, while not affecting the bulk properties of the etch barrier that are critical for pattern integrity. This surfactant layer can also serve as a buffer layer that protects the FSAM treatment by minimizing its interaction with the bulk etch barrier.
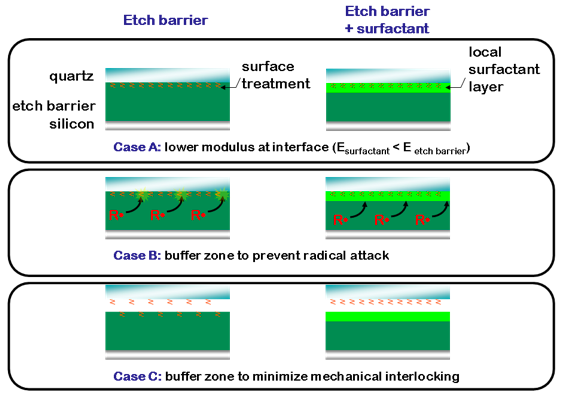
Figure 7. Surfactants migrating to the template-polymer interface provide opportunities to lower the adhesive force: Case A) a low modulus layer helps facilitate separation; Case B) an inert buffer layer minimizes exposure to radicals, which can attack the FSAM; Case C) a local layer of low modulus decreases the adhesion contribution from mechanical interlocking.
Two different surfactants were added (1 to 5 wt% concentration) to a standard etch barrier formulation. Surfactant R is a solid acrylated material that is able to react with the polymer matrix during photopolymerization, while surfactant NR is a volatile liquid that does not react.
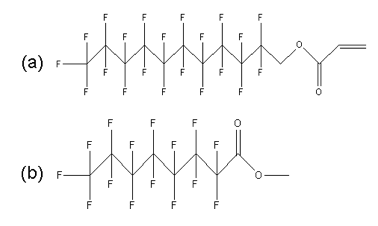
Figure 8. Fluorinated surfactants a) 2-(Perfluorodecyl)ethyl acrylate (reactive), b) methyl perfluorooctanoate (nonreactive).
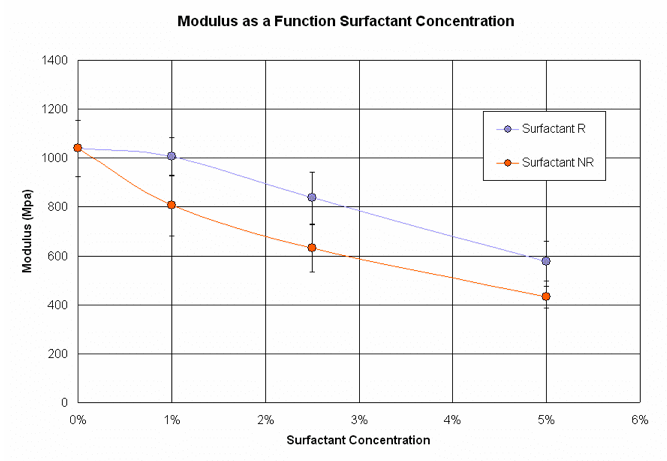
Figure 9. Tensile experimental results showing modulus as a function of surfactant concentration.
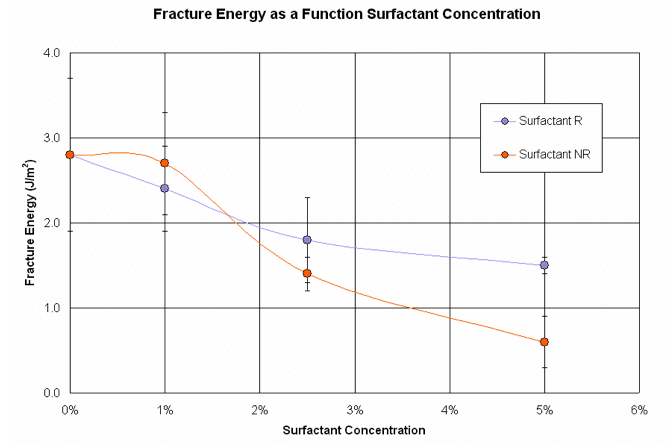
Figure 10. Four-point bend results showing fracture energy as a function of surfactant concentration.
As show in Figures 9 and 10, both surfactants significantly decrease the modulus and fracture energy at 5 wt% loading. Surfactant NR is more effective than surfactant R in reducing both of these parameters.
Using angle-resolved XPS, we are able to track the migration of these surfactants to determine what template surface treatments are ideal for this process. The tested surface treatments include the following: fluorinated self-assembled monolayer (FCDS), bare quartz (no treatment), diamond-like carbon (DLC), and no template (air).
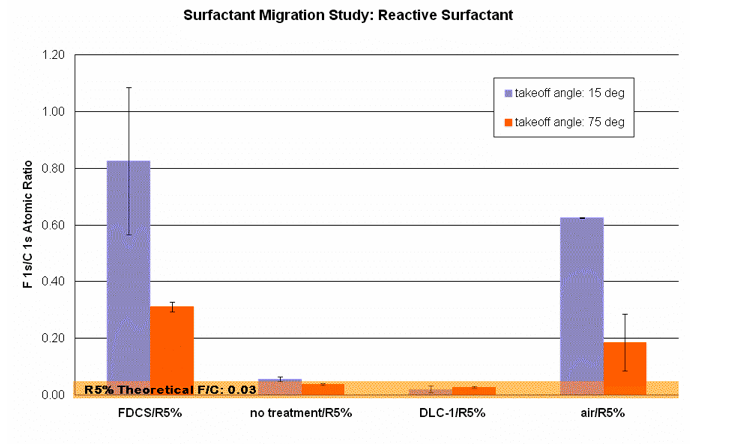
The fluorine to carbon atomic ratio was measured at 2 nm (15° takeoff angle) and 7 nm (75° takeoff angle) into the film. These data were plotted with the theoretical F/C ratio, which assumes the surfactant is uniformly distributed in the polymer matrix. According to Figure 11, both FDCS and air interfaces yield F/C atomic ratios above the theoretical bulk value, providing strong evidence for migration. A concentration gradient also exists in these two samples as evidenced by the fact that F/C ratios at 15° are higher than at 75°. On the other hand, both untreated quartz and DLC appear to either have no effect on surfactant migration or actively repel the surfactant from the surface. Based on these results and our observations, we conclude that the use of fluorinated surfactants must be accompanied by a low energy surface to induce migration.
Continue reading >> Miscellaneous Studies