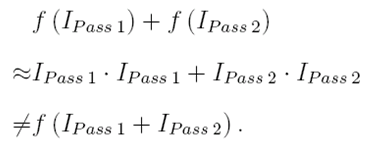Potential Materials
Several materials have been proposed to implement a nonlinear response to exposure and theoretically permit double exposure pitch doubling including contrast enhancement layers (CELs), two-photon materials, intermediate state two-photon (ISTP) materials, and optical threshold layers (OTLs). These materials and their theory of operation are described in the following sections.
Contrast Enhancement Layers
Contrast enhancement layers (CELs) are strongly absorbing materials that increase transparency, or photobleach when exposed to light. A CEL is normally applied directly on top of the resist layer. During exposure, energy is first devoted to photobleaching the CEL. As the CEL becomes transparent, the energy is then able to reach the resist and initiate the solubility switch. Light can only penetrate through the CEL in regions where aerial image intensities are high (non-opaque regions on the mask) and cannot reach the resist in regions where aerial image intensities are lower (opaque regions on the mask). This introduces a nonlinear transfer of the applied aerial image into the photoresist and improves the resolution. CELs can be divided into two different subtypes, namely, reversible (rCEL) and irreversible (irCEL). The main difference between the two subtypes is that, in rCELs, the photobleached regions can return to the initial opaque state between exposure passes whereas in irCELs, the photobleaching is irreversible.
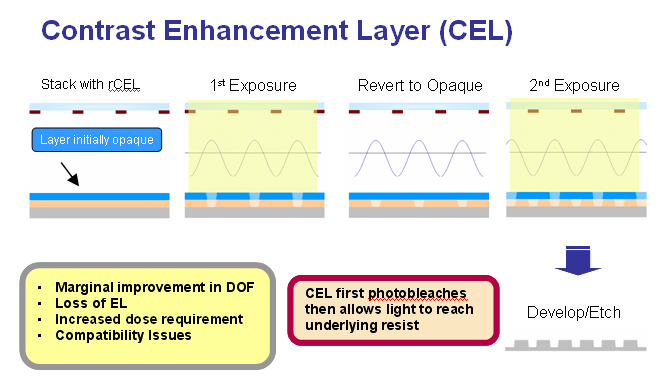
We have investigated the use of rCELs with 50 nm lines/space patterns in single exposure mode. The results are shown in the following figures.
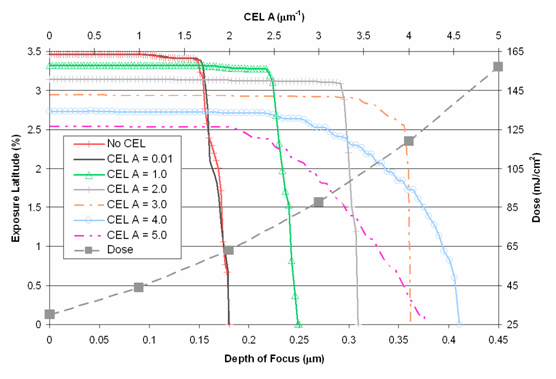
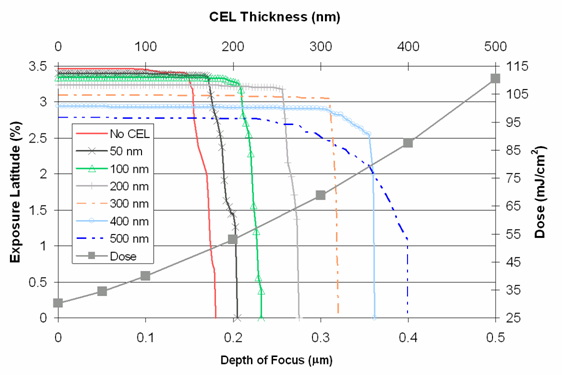
The figures show that the use of the rCEL moderately increased the depth of focus for the process, but the increase comes at the cost of increases in exposure doses, almost by a factor of 10 in the case of A = 5.0 Μm-1 when compared to the No CEL case, and in most cases decreases in EL.
Two-Photon Materials
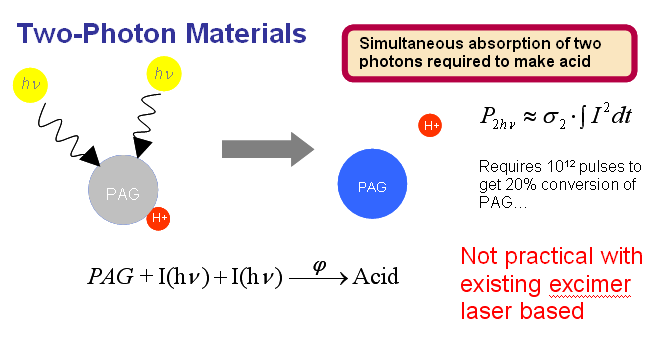
Two-photon photoresist systems involve the incorporation of photoacid generators (PAGs) that require the simultaneous absorption of two photons to induce the photochemical acid generation. The chemical reaction for a two-photon photoacid generator can be described by the following reaction
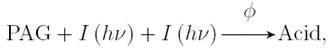
where Φ is the quantum efficiency of the two-photon photochemical reaction. Since the simultaneous absorption of two photons is required for the reaction, the probability of conversion is proportional to the light intensity squared, which provides a nonlinear response to exposure energy
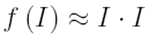
Unlike the CEL, two-photon materials are not enhancement layers that are applied on top of existing resists, but rather the nonlinear response is incorporated directly into the resist formulation. This eliminates complexities introduced by the addition of an extra film layer such as depth of focus and material compatibility.
High efficiency two-photon PAGs have not yet been developed to work with 193 nm. Analysis of the two-photon reaction kinetics suggests that a very large increase in exposure source output would be required to produce a viable level of process throughput. Therefore, without a very large increase in exposure source intensity, the existing two-photon PAG materials could not maintain the throughput level typical of current lithographic process.
Intermediate State Two-Photon Materials
Intermediate state two-photon (ISTP) layers are materials that generate acid molecules in a two step process. Similar to two-photon materials, ISTP materials alter the acid generation behavior of the resist medium. Although each step requires the absorption of a photon, ISTP materials are not true two-photon processes in that the acid production does not have a quadratic dependence on dose. The reaction sequence is
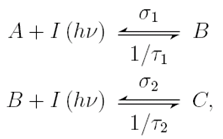
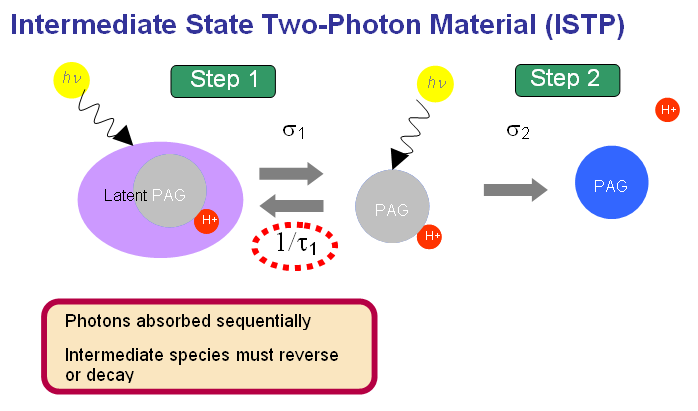
Although ISTP materials do not exhibit true two-photon behavior, they may require significantly lower doses to generate acid compared to two-photon resists. The trade off between lower reaction orders may be offset by the lower doses.
The behavior of ISTP materials depends on the ability of the intermediate species to revert to the initial state. A build up of the intermediate species will effectively render the sequence to become a first ordered reaction that is controlled by a rate limiting step. Therefore, the characteristics of the exposing laser such as the energy per pulse (A0), pulse cycle time (tf, also the inverse of the repetition rate), and full width half max (FWHM) also have to be considered.
Optical Threshold Materials
Optical threshold layers (OTLs) are materials that require the absorption of a threshold exposure dose to induce a photochemical event. The exposure threshold gives the material a region of nonlinear response to exposure dose and allows OTLs to be used as double exposure resists. Nonlinearity derives from the fact that any dose absorbed below the threshold does not cause reactions to occur.
Above the dose threshold, a step change in conversion occurs and is described by the following piecewise function
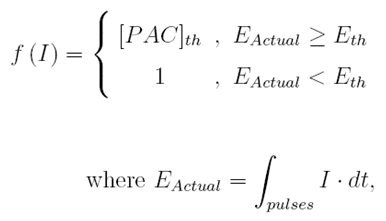
EA and Eth represent the actual dose received by the material and the threshold dose, respectively. [PAC]th is the step wise conversion concentration of the photoactive compound after reaching Eth.
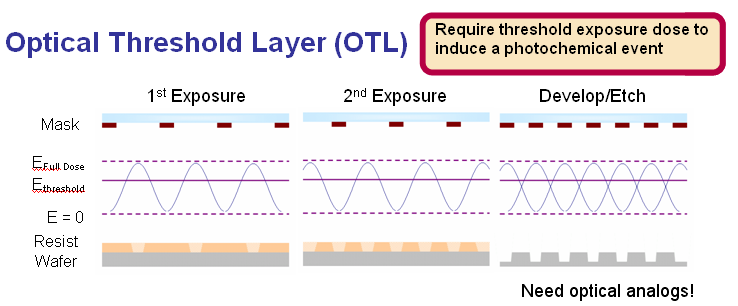
Analogous thermal resist systems are already in use in the printing industry. Thermal resists rely upon a thermal image instead of an optical image. The thermal image is derived from the absorbance of high intensity light images. Thermal resists use the absorbed thermal energy to induce a phase change in the system and cause the solubility switch as opposed to traditional chemical resists which use the energy to carry out a chemical reaction. Phase changes are ideal as the dose dependent mechanisms in OTL materials because the threshold response is inherently built into the thermodynamics of the system. For example, in the liquid to vapor transition of water, the thermal dose required to vaporize the water is the sum of the energy required to bring the water from the initial temperature to the vaporization temperature and the latent heat of vaporization. Below the threshold dose, the transition does not occur. The system also does not have any memory effects to the thermal dose as the thermal dose required for vaporization per unit mass at a given initial temperature remains constant even after multiple heating and cooling cycles.
Analogous chemical systems will most likely also need to use the optical image to induce a phase change in the material which will then allow a solubility switch. The phase change will replicate the behavior of thermal resists in that the absorption of a threshold dose is required to induce phase change. Below the threshold, no solubility switch should occur and the material should not have resist memory.
Continue Reading >> Simulation