Experimental
The evaluation of the materials is to prove if it has a reversibly photoswitchable permeability. The following procedures are required to achieve the evaluation.- Isomerization of azo polymer
- Reversibly photoswitchable melting point of the azo polymer thin film
- Permeability measurements of the polymer thin film
Isomerization of azo polymer
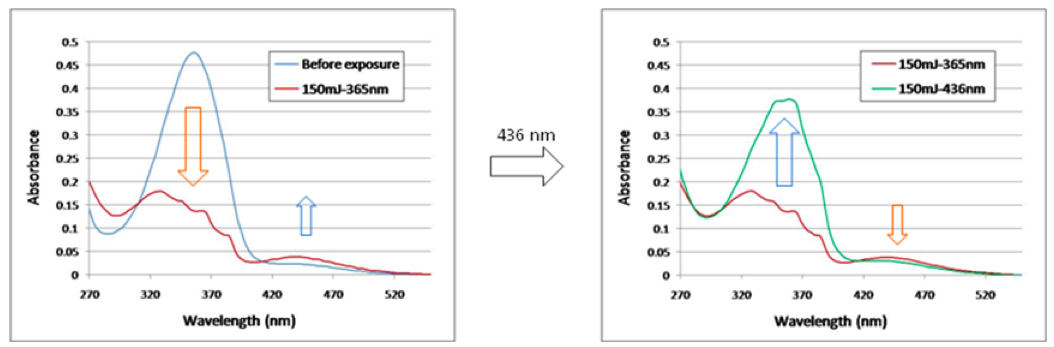
The isomerization of the azo polymers that we made was confirmed by UV-VIS. The following figure reprents the UV-VIS of one of our azo polymers. The peak (358 nm) is the absorption of the trans- form of the azo group while the peak (438 nm) is that of the cis- form. The 358 nm peak goes down upon the exposure of the 365 nm UV light. Meantime, the 438 nm peak goes up. With the exposure of the 436 nm UV light, these peaks behave the other way round.
Reversibly photoswitchable melting point of the azo polymer thin film
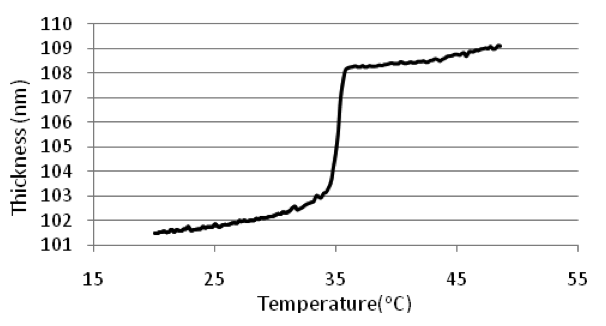
The melting point of the polymer thin film was measured by ellipsometry in terms of the thermo expansion. The thickness of the polymer thin film is proportional to the temperature without changing the phase. There is a jump in the thickness-temperature function through melting. It is shown below. This jump can be used to indicate the melting point of the polymer thin film.
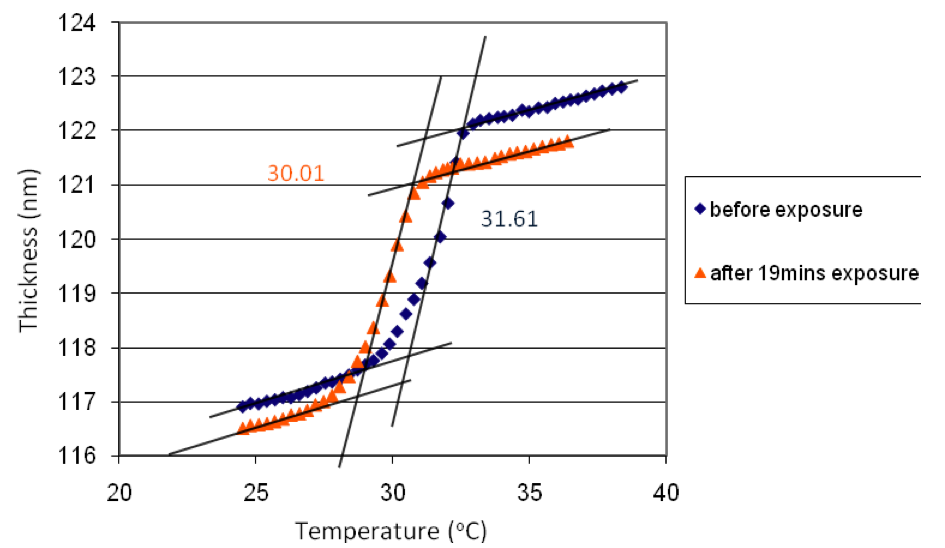
Once the melting point of the polymer thin film was obtained by ellipsometry, the same polymer thin film was exposed at 365 nm. The azo groups in the polymer isomerized and caused the decrease of the melting point by 1.60 oC as shown below.
Permeability measurements of the polymer thin film
The permeability of the polymer thin film is crucial to the OPT process. It is the main control of the image. The permeability of the thin film is measured according to the Fickian's Equation
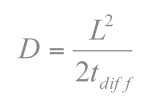
where L is the diffusion length and tdiff is the diffusion time. In terms of this equation, the experiment was designed by the following setup. Tri-layer film stack can be coated as a film sample. The bottom layer is the acid feeder layer while the top layer is the acid detector layer. The middle layer is the diffusion barrier layer which is the azo polymer. The acid feeder we chose was a simple 248 nm PAG (Triphenol sulfonium triflate). The acid detector was PTBOCST which can react with acid above 70oC. The acid-catalyzed reaction is the deprotection of the tert-butoxycarbonyl (TBOC) group which can be detected by FTIR since the carbonyl group has a strong absorption peak at 1750 cm-1.
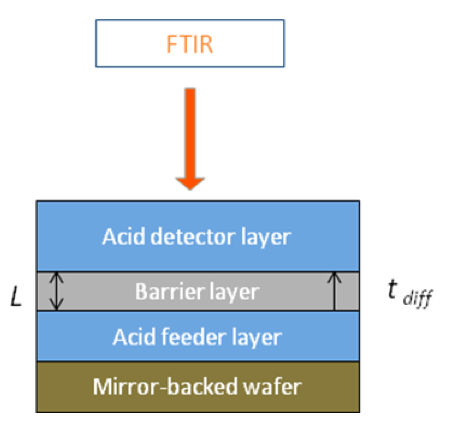
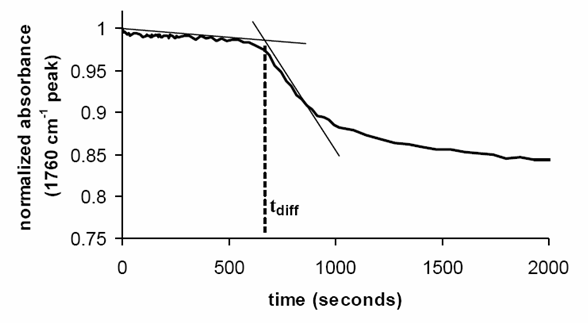
In this experiment, the diffusion specie is the triflic acid and the diffusion length is the thickness of the barrier layer. The diffusion time is how long the triflic acid takes to achieve the bottom of the acid detector layer. The sample result is shown below. Once the acid reaches the bottom of the PTBOCST layer, it reacts with the polymer and starts to deprotect the hydroxystyrene. So, the intensity of the carbonyl group starts to decrease at that moment which indicates the diffusion time.
Sergei, UT Dissertation
Collaborations