Release Layer |
| It is imperative, following
exposure, that the polymerized etch barrier adhere to the underlying
transfer layer, and release easily and completely from the template. The
successful release of the imprint template from the imprinted polymer
depends in part on the surface energies of the template. Hare, et al. predicted that a surface composed of only -CF3
groups would have the lowest critical surface tension of any system, ~6
dynes/cm.1 Nishino, et al. later
verified that prediction, and demonstrated a surface energy of 6.7
dynes/cm for C20F42 adsorbed on pretreated glass.2 Diffraction
studies indicated that the C20F42 chains were
aligned perpendicular to the glass substrate, which exposed -CF3
end groups on the sample surface. As a comparison,
TEFLON®,
known for its release properties, has a surface energy of 18 dynes/cm.3
A monolayer film with -CF3 terminations
clearly has the potential to be a superb release coating for the SFIL
template. A fluoroalkyltrichlorosilane precursor is used to form such a
monolayer on the template surface to promote this release.
4,5
|
Liquid phase and vapor
phase application methods have been studied.
In
both cases, The –OH groups on the SiO2 surface react with the
trichlorosilane, forming HCl as a byproduct.6
The reaction of alkyltrichlorosilanes with SiO2 proceeds very
slowly in the absence of surface water, but quite rapidly in the
presence of surface water.7-10 Tripp and Hair studied the reaction of
alkylchlorosilanes and fluoroalkylchlorosilanes with silica by
monitoring the SiOH groups with infrared spectroscopy. They found that
alkylchlorosilanes did not react with a completely dehydrated surface,
and the fluorinated counterparts reacted slowly.
In the presence of surface bound water, the precursors react readily to
form networks derived from the formation of bonds between adjacent
molecules. The water reacts with the self-assembled monolayer (SAM)
precursor to form a silanol intermediate and acid in an irreversible
reaction:11 |
|
R-SiCl3 + 3H2O
Þ
R-Si(OH)3 + 3HCl |
|
The intermediate has three -OH groups, which can either bond to the
quartz surface or to adjacent molecules through the loss of water. This
network formation, pictured in Figure 1, should lead to a durable release coating.
Following the SAM formation, there may be some dangling -OH groups on
the substrate surface as well as in the film. Tripp and Hair have shown that post-formation annealing enhances the
incorporation of these groups into the bonding network.12
|
 |
| Figure 1.
Self-assembling monolayer formation. A fluorinated self-assembling
monolayer is used to provide a low surface energy coating that ensures
release of the cured etch barrier. a) Alkyltrichlorosilanes migrate to
the template surface, where they react with surface water to form
silanol intermediates. b) These intermediates undergo condensation
reactions with hydroxyl groups on the quartz surface, as well as those
on adjacent silanols, to form a networked siloxane monolayer. The
orientation of the chains is away from the quartz, in a 3-D comb-like
structure. Annealing further enhances the condensation, creating a
highly networked, durable, low surface energy coating. |
| In one set of experiments
the quartz templates were cleaned in an acetone ultrasonic bath,
followed by O2 RIE at 50 W, 10 sccm O2, 20 mTorr
for 10 min to remove organic contaminants on the surface. The clean
templates were then treated with a release agent,
tridecafluoro-1,1,2,2-tetrahydrooctyltrichlorosilane (Gelest), by vapor
exposure at 1 atm total pressure (precursor plus N2) for 90
min at room temperature, and annealed at 100 ºC for 15 min. This surface
treatment reaction has yielded surface energies in the neighborhood of
12 dynes/cm.5 |
| The surface treatment must
maintain its release characteristics through thousands of imprints in a
manufacturing process. Preliminary results indicate that the current
technique could provide films with the required durability.
Figure 2 shows the water contact angle on an
untreated template (left), a newly treated template (center), and a
treated template (right) that was used for a period of two months and
was cleaned vigorously. The template retained its release functionality,
and it can be seen that the water contact angle did not change
significantly. We have seen no evidence of catastrophic loss of release
function, and work is currently underway to quantify film durability for
a variety of surface treatment conditions. |
 |
| Figure 2. Water
contact angle on an untreated template (left), a newly treated template
(center), and a treated template (right) that was used to imprint
numerous times. The template retained its release functionality, and it
can be seen that the water contact angle did not change significantly. |
| XPS analysis
of the C region indicates that the film consists of as semi-fluorinated
chain, and the C peak distribution is as would be expected from a film
formes using this precursor, as shown in Figure 3. |
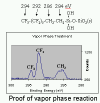 |
| Figure 3. XPS
spectrum (C region) showing peak splitting in agreement with a film of
FOTS molecules. |
| There are presumably ideal processing
conditions that yield maximum coverange and surface bonding, leading to
very durable films. Current work is oriented at finding those ideal
conditions for the current reaction scheme. |
| Also see Interfacial Adhesion Studies |
References |
-
E.F. Hare, E.G.S., W.A. Zisman, J. Phys Chem., 1954.
58: p. 236.
-
T. Nashino,
M.M., K. Nakamae, M. Matsushita, Y. Ueda. Langmuir, 1999. 15:
p. 4321-4323.
-
Ulman, A.,
An Introduction to Ultrathin Organic Films from Langmuir-Blodgett to
Self-Assembly. 1991, Boston: Academic Press. 442.
-
Colburn,
M., et al., Proc. SPIE: Emerging Lithographic Technologies III,
1999. 3676(I): p. 379.
-
Bailey,
T., et al., J. Vac. Sci. Tech. B, 2000. 18(6): p. 3572.
-
Tripp, C.P. and M.L. Hair. Langmuir, 11 (1995) 1215.
-
X. Zhao,
R.K. J. Phys. Chem., 1996. 100: p. 11014-11018.
-
P. Silberzan, L.L., D. Ausserre, J.J. Benattar.
Langmuir, 1991. 7: p. 1647-1651.
-
J.D. Le Grange, J.L.M.. Langmuir, 1993.
9: p. 1749-1753.
-
C.P. Tripp, R.P.N.V., M.L. Hair.
Langmuir, 1993. 9: p. 3518-3522.
-
C.P.
Tripp, M.L.H. Langmuir, 1995. 11: p. 1215-1219.
-
C.P.
Tripp, M.L.H. Langmuir, 1995. 11: p. 149-155.
|



