Etch Barrier |
| The etch
barrier material is subject to several design constraints, some of which
are summarized in Figure 1. The etch
barrier liquid must be dispensable from an automatic fluid dispense
system, and must not change significantly in composition between
dispensing and imprinting by, e.g., component evaporation. It must be
readily displaced during the imprint step and photopolymerize rapidly
during exposure. Shrinkage due to polymerization must be controlled. The
polymer must release from the template while adhering to the transfer
layer, and it must exhibit sufficient rigidity to avoid feature collapse
It must exhibit some level of temperature stability to withstand the
etching temperatures, and it must exhibit sufficient etch selectivity
during the O2 RIE step to allow for high aspect ratios to be generated
in the transfer layer. |
 |
|
Figure 1. Summary of some etch barrier
requirements. |
| The SFIL
process relies on photopolymerization of a low viscosity, acrylate-based
solution. Acrylate polymerization is known to be accompanied by
volumetric shrinkage that is the result of chemical bond formation.
Consequently, the size, shape, and placement of the replicated features
may be affected. Volumetric shrinkage was found to be less than 10%
(v/v) in most cases.1,2 |
| Finite element
modeling (FEM) results indicate that no significant pattern placement
distortion will result as a consequence of densification. Additionally,
the sidewall angle is predicted to be close to 90° for high aspect ratio
features, and will approach 80° for low aspect ratio features, using the
worst-case model parameter of 17% (v/v) densification.1,2 |
| In separate
studies of various acrylate and methacrylate monomers, the effect of
fluence was examined using IR to follow the isolated C=C stretching
absorbance peak at 1640 cm-1 and revealed nearly complete conversion
with less than 30 mJ/cm2.3 Further information on UV curing of acrylate
coatings can be found in works by Decker4
and Kloosterboer.5-7 |
| The current
etch barrier liquid is a multicomponent solution. The silylated monomer provides etch resistance in the O2
transfer etch. Crosslinker monomers provide thermal stability to the
cured etch barrier and also improve the cohesive strength of the etch
barrier. Organic monomers serve as mass-persistent components and lower
the viscosity of the etch barrier formulation. The photoinitiators
dissociate to form radicals upon UV irradiation, and these radicals
initiate polymerization. The current formulation includes the monomers
whose structures are shown in Figure 2. |
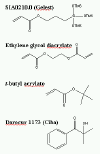 |
| Figure 2. Chemical structures of current etch
barrier monomers. |
-
Colburn, M., et al., J. Vac. Sci. Tech. B., 2001. 19(6):
p. 2685.
-
Colburn, M.E., Step and Flash Imprint Lithography: A
Low-Pressure, Room-Temperature Nanoimprint Lithography. Department
of Chemical Engineering; Ph.D. Thesis. 2001, Austin, TX: The
University of Texas at Austin.
-
Colburn, M., et al., Proc. SPIE: Emerging
Lithographic Technologies III, 1999. 3676(I): p. 379.
-
Decker, C., Prog. Polym. Sci., 1996. 21: p. 593.
-
Kloosterboer,
J.G. and G.J.M. Lippits, J. Imaging Science, 1986. 30(4): p.
177.
-
Kloosterboer,
J.G. and G.F.C.M. Lijten, Polymer, 1987. 28(7): p. 1149.
-
Kloosterboer, J.G., G.F.C.M. Lijten, and C.P.G. Zegers,
Polym. Mater. Sci. Eng., 1989. 60: p. 122.
|


