Process & Projects
Decoupling Gain from Blur in Chemically Amplified Resists
The polymer, poly(phthalaldehyde) (PPHA), has an extremely low ceiling temperature, and we have discovered that it can be used as a dissolution inhibitor for poly(norboronenehexafluoroalcohol) (PNBHFA).
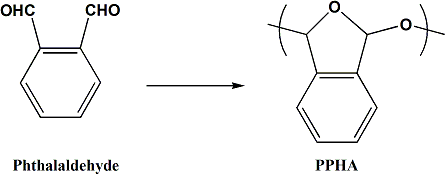
Blends of the polymers containing small amounts of PPHA are insoluble in base developer. If this dissolution inhibitor is exposed to acid, it spontaneously unzips which allows the exposed areas of the PPHA-PNBHFA blend to dissolve in the developer solution. Preliminary studies using PAG as the initiator have shown the unzipping concept works, and images have been created in such a polymer blend.
To decouple the gain from the blur, PPHA was end capped with 2-nitrobenzaldehyde. The nitro- group in the ortho- position allowed the polymer to unzip upon exposure to 365 nm light via a well documented photodecomposition. In this way, the gain derives from the fact that a single photochemical event causes a large number of chemical reactions. The quantum efficiency for bond breaking is equal to the quantum efficiency for the photoscission multiplied by the degree of polymerization.
On the other hand, the gain is localized on a single chain; there is no interchain transfer of reactivity. This makes the maximum blur the diameter of the sphere of gyration of just one of the dissolution inhibitor polymers compared to the PAG system whose blur is the length of diffusion of the acid in the resist.
This new technique demands phase compatibility between the PNBHFA and the dissolution inhibitor. That was achieved by limiting the degree of polymerization of the PPHA, but nevertheless this was an effective proof of principle experiment and shows extreme potential for creating a sensitive system that does not have the bias and LER problems created by the classical chemical amplification design. I will be synthesizing several different phthaldaldehyde analogs and different initiators and end-capping molecules designed to improve the phase compatibility between the PNBHFA and the dissolution inhibitor and to optimize the contrast of the resist.
Non-Chemically Amplified Resists
Poly(methylmethacrylate) (PMMA) has long been the workhorse resist for electron beam (e-beam) mask writing because its etch resistance and resolution are acceptable for current processing requirements. However because the system is not chemically amplified, the exposure time is much longer than the chemically amplified systems. E-beam mask writers are tremendously expensive to purchase and maintain, so throughput of product is a key issue to this industry. Nippon Zeon Company is producing a relatively new non-chemically amplified e-beam resist (ZEP-520), which is a copolymer of α-chloromethacrylate and α-methylstyrene.
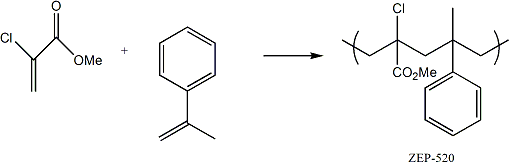
The addition of the chloro- group increases the sensitivity of this polymer to the electrons for polymer backbone scission. However, this substituent also under goes dissociative electron capture leading to a loss of HCl and production of crosslinks within the resist, a reaction which is clearly counter productive.
To improve the non-chemically amplified mask resists, we propose to replace the α-chloro group with an α-trifluoromethyl group, which should preclude dissociative electron capture and crosslinking but still augment the main chain scission efficiency and thereby reduce the dose time required for imaging. We therefore propose to synthesize a homopolymer of α-trifluoromethylmethacryalte and a copolymer of α-trifluoromethylmethacryalte and α-methylstyrene. These materials will be tested as high resolution, high throughput e-beam resists.
