Synthesis and Characterization of Norbornane Diol Isomers and Their Fluorinated Analogs |
| The continued drive to miniaturize microelectronic devices has been accomplished by printing the circuit elements with ever decreasing wavelengths of light.[1] Leading edge manufacturing is now done with 193nm light, and many are pursuing exposure at 157nm. |
| Polynorbornenes are of particular interest as the basis for the design of photoresists due to their unique set of desirable properties including: high glass transition temperatures, high resistance to reactive ion etching, low UV absorbance, and relative ease of synthesis.[2] |
| However, at 157nm, even aliphatic hydrocarbons such as polynorbornene absorbs strongly.[3] Fortunately, introduction of fluorinated substituents into the norbornane skeleton provides material with sufficient transparency to be used at this wavelength[4], but the most transparent of the fluorine substituted norbornene monomers resists addition polymerization initiated by classical catalysts.[2] |
| Therefore an alternate route to fluorinated norbornene polymers by condensation polymerization was sought. Norbornane diols offer a number of attractive opportunities for the preparation of such polymers, for example via condensation with phosgene to yield polycarbonates. |
| Although synthetic routes from norbornene to the 2,3-dihydroxy[5] and 2,7-dihydroxy[6][7] norbornane have been reported, much of this chemistry is not viable for fluorine substituted norbornane systems as the synthetic methods do not provide sufficient yields or purity of the monomers. The preparation of fluorinated norbornane compounds most easily proceeds through the Diels-Alder reaction of cyclopentadiene with a fluorinated alkene, which have been previously investigated.[4] |
| In this study norbornene was used as a model to develop routes to the corresponding diols. After the synthesis was optimized for norbornene, these routes were investigated with the incorporation of fluorinated substituents. It was soon discovered that in most cases, the hydrocarbon norbornene was a not an appropriate model for the fluorinated monomers. |
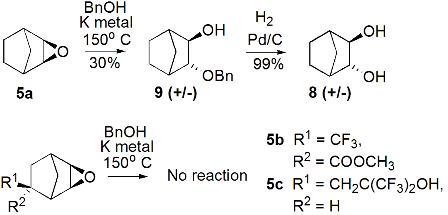 |
| SCHEME 1: Base catalyzed 2- exo-3-exo-epoxynorbornane ring opening products. |
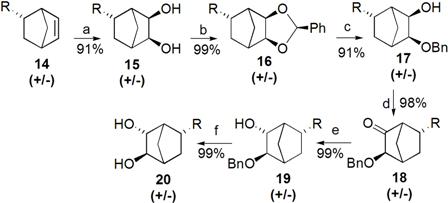 |
| SCHEME 2: Preparation of fluorinated norbornane monomer. Key: R = CH2C(CF3)2OH (a) OsO4, NMO, THF; (b) PhCH(OCH3)2, PhCH3, 0 °C, 10 Torr; (c) DIBAL, PhCH3 (d) PCC, CH2Cl2; (e) superhydride, THF 0 °C; (f) Pd/C, H2, EtOH/EtOAc |
References |
| 1. Levinson, H. J. Principles of Microlithography, 2nd ed.; SPIE Press: Bellingham, WA, 2005. |
| 2. Saunders, D. P.; Connor, E. F.; Grubbs, R. H.; Hung, R. J.; Osborn, B. P.; Chiba, T.; MacDonald, S. A.; Willson, C. G.; Conley, W. Macromolecules, 2003, 36, 1534; Barnes, D. A.; Benedikt, G. M.; Goodall, B. L.; Huang, S. S.; Kalamarides, H. A.; Lenhard, S.; McIntosh, L. H.; Selvy, K. T.; Shick, R. A.; Rhodes, L. F. Macromolecules 2003, 36, 2623; Benedikt, G. M.; Elce, E.; Goodall, B. L.; Kalamarides, H. A.; McIntosh, L. H.; Rhodes, L. F.; Selvy, K. T.; Andes, C.; Oyler, K.; Sen, A. Macromolecules 2002, 35, 8978; Lipian, J.; Mimna, R. A.; Fondran, J. C.; Yandulov, D.; Shick, R. A.; Goodall, B. L.; Rhodes, L. F.; Huffman, J. C. Macromolecules 2002, 35, 8969; Mathew, J. P.; Reinmuth, A.; Melia, J.; Swords, N.; Risse, W. Macromolecules 1996, 29, 2755. |
| 3. Kunz, R. R.; Bloomstein, T. M.; Hardy, D. E.; Goodman, R. B.; Downs, D. K.; Curtin, J. E. Proc. SPIE 1999, 3678, 13. |
| 4. Trinque, B. C.; Chambers, C. R.; Osborn, Br. P.; Callahan, R.P.; Lee, G. S.; Kusumoto, S.; Sanders, D. P.; Grubbs, R. H. Conley, W. E.; Willson, C. G.J. Fluorine Chem. 2003, 122, 17; Tran, H. V.; Hung, R. J.; Chiba, T.; Yamada, S.; Mrozek, T.; Hsieh, Y.-T.; Chambers, C. R.; Osborn, B. P.; Trinque, B. C.; Pinnow, M. J.; Sanders, D. P.; Connor, E. F.; Grubbs, R. H.; Conley, W.; MacDonald, S. A.; Willson, C. G. J. Photopolym. Sci. Technol.2001, 14, 669. |
| 5. Heyns, K.; Rudiger, G.; Paulsen, H. Chem. Ber. 1972, 105, 1019; Taylor, J. E. Synthesis 1985, 1142. |
| 6. Kwart, H.; Vosburgh, W. G. J. Am. Chem. Soc. 1954, 76, 5400. |
| 7. Walborsky, H. M.; Loncrini, D. F. J. Am. Chem. Soc. 1954, 76, 5396; Crandall, J. K. J. Org. Chem.. 1964, 29, 2830; Shealy, Y. F.; Clayton, J. D. J. Am. Chem. Soc. 1969, 91, 3075; Tenaglia, A.; Terranova, E.; Waegell, B. Tetrahedron Lett. 1990, 31, 1157; Kotsuki, H.; Kataoka, M.; Nishizawa, H. Tetrahedron Lett. 1993, 34, 4031. |
Version History |
| Original page created 02/27/06 |
