| Fluorocarbon Backbone Module |
|
Our research group has placed a large amount of focus on cabon-based polymers for
the backbone module of 157 nm photoresists. The use of these materials holds an
advantage over silicon-based materials since it allows the extension of existing
DUV or 193 nm photoresist polymers to 157 nm designs. Unfortunately, studies have shown that
in existing photoresist materials absorb strongly at this wavelength (1), and
that only through strategic placement of electron-withdrawing substituents can
this absorbance be attenuated (see etch resistance
section). |
| Acrylates |
|
Acrylate polymers have been used extensively in 193 nm photoresist
designs due to their facile functionalization and favorable polymerization
characteristics(2). Unfortunately, the p-system in these polymers
contributes a large amount of absorbance at 157 nm and effectively precludes
their use at this wavelength. However, our research group has recently shown the absorbance of these
materials can also be significantly decreased through the addition of
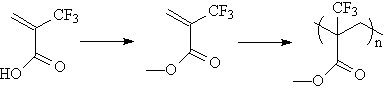
electron-withdrawing substituents(3). For example, poly(methyl trifluoromethacrylate) was prepared from the
commercially available acid(4), and polymerized according to literature
procedures(5). |
 |
|
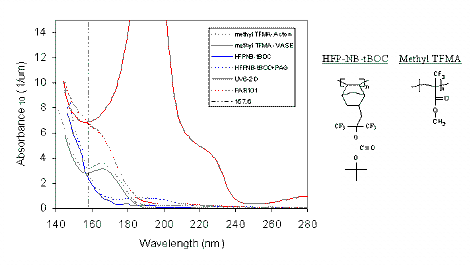
The absorbance spectrum of the final homopolymer is displayed below, showing that this material has an absorbance of ~3 / mm, less than half that of PMMA. |
 |
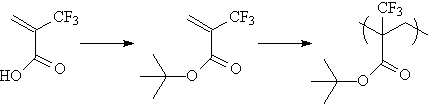
| To confirm that the favorable imaging characteristics of acrylates
has not been adversely affected, poly(tert-butyl trifluoromethacrylate)
was prepared(6) and is undergoing preliminary imaging experiments. |
 |
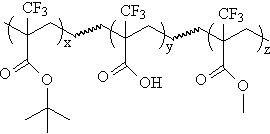
| Although the results from these experiments will give some
indication of the potential performance available from this class of materials,
a candidate for an actual photoresist polymer will most likely have to follow a
model similar to that described for acrylates as 193 nm photoresists(2). To that end, we are in the process of
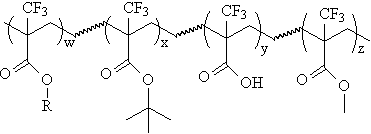
preparing the acrylate ter- and tetrapolymers shown below to determine their
imaging potential. |
 |
 |
|
R=Fluorinated alicyclic unit for etch-resistance (see etch resistance section) |
| Vinyl addition polymerization of alicyclics |
|
Our project of systematically replacing hydrogen with fluorine on
alicyclic units to their decrease absorbance is an ongoing project (see etch resistance section).However, preliminary results are encouraging enough to indicate that alicyclic units might be useful
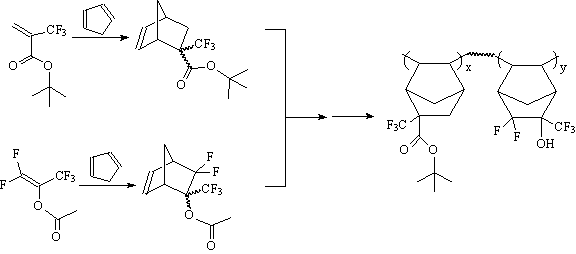
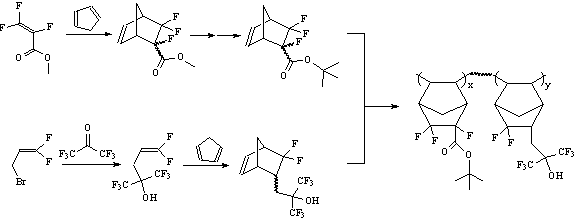
in 157 nm photoresist designs. Promising alicyclic monomer units can be readily prepared from the
fluorinated acrylates described in the previous section. Additional alicyclic monomers can be
envisioned from other fluorinated acrylates and olefins such as the
pentafluoroisopropenyl acetate(7) or 3-bromo-1,1-difluoro-propene(8) shown
below. This provides a pool of potential monomers to produce promising photoresist polymer candidates
via transition metal-catalyzed vinyl addition polymerization. Several monomers have been prepared and
experiments to produce polymers are underway. |
 |
 |
| Free radical polymerization of alicyclics |
| A second method of polymer formation with alicyclic monomers is
through copolyermization with electron-poor comonomers such as malice
anhydride. This was the basis of our research group’s 193 nm photoresist platform(9). Unfortaunely, such materials demonstrate
high absorbance at 157 nm due to both the alicyclic and carbonyl subunits in the
repeat unit. However, we have shown
that the absorbance of the alicyclic unit can be attenuated by the addition of
fluorine. We hope to apply the same
method to decrease the absorbance of the maleic anhydride comonomer to allow
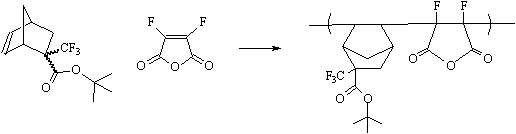
this polymer class to be used as 157 nm photoresists. The preparation of difluoromaleic
anhydride has been previously reported(10), and we are currently working to form
the promising copolymer shown below. |
 |
|
1) Bloomstein, T. M., Horn, M. W., Rothschild, M., Kunz, R. R., Palmacci, S.
T., Goodman, R. B., “Lithography with 157 nm Lasers”, J. Vac. Sci. Technol. B, 1997,
13(6), 2112. |
|
2) Allen, R. D., Wan, I. Y., Wallraff, G. M., DiPietro, R. A., Hofer, D. C.,
Kunz, R. R. “Microelectronics Technology; Polymers for Advanced Imaging and
Packaging” American Chemical Soceity, Washington D.C., 1995, Ch. 17. |
|
3)Conley, Will Presentation, International Symposium on 157nm Lithography,
Dana Point, California, 2000. |
|
4) C. Botteghi, C. Lando, U. Matteoli, S. Paganelli, G. Menchi, J. Fluorine Chem. 1997, 83(1), 67 |
|
5)H Ito, ACS Symposium Series 696, “Application of anionic polymerization research” 1996, 218. |
|
6)A. L. McCloskey, G. S. Fonken, R. W. Klüber, W. S. Johnson, Org. Synth. Coll. Vol. IV, 1963, 261 |
|
7)Y. V. Zeifman, S. A. Postovoi, L. S. German, Russ. Chem. Bl. 1994, 43(1), 170. |
|
8)H. Muramatsu, P. Tarrant, J. Org. Chem. 1964, 29, 1796. |
|
9)K. Patterson, U. Okoroanyanwu, T. Shimokawa, S. Cho, J.D. Byers, C.G. Willson, "Improving the Performance of 193 nm Photoresists Based on Alicyclic
Polymers", Proc. SPIE, Adv. in Resist Technology and Processing, 1998, 3333, 425. |
|
10)Krespan, U. S. Patent #5,112,993, 1992. |








