| Etch Barrier Module |
| The etch barrier module is responsible for imparting resistance to plasma etching conditions to the overall polymer. As discussed in the first chapter, etch resistance has been empirically linked to the ratio of the atomic components of the photoresist polymer repeat unit (1). This empirical model offers varying levels of predictive ability depending upon the nature of the etching plasma utilized for evaluation. Nonetheless, this model predicts that structures with high degrees of unsaturation, achieved through the use of either double bonds or cyclic structures, should offer the optimum level of etch resistance. The aromatic units present in 248 nm photoresist polymers present a high degree of unsaturation due to the three double bonds and one ring that comprise each phenyl group. These functionalities are therefore well suited to provide resistance to plasma etching conditions. Unfortunately, all aromatic materials evaluated in the surveys carried out at MIT Lincoln Labs have offered high absorbance values at 157 nm. |
| As described in chapter 1, aromatic groups also contribute a significant amount of absorbance at 193 nm. As a result, these functionalities were excluded from 193 nm photoresist polymer designs and replaced exclusively with alicyclic units to provide the necessary plasma etch resistance. A similar approach is likely to be followed for 157 nm photoresist polymer designs. However, due to the rather large absorbance contributions presented by most hydrocarbon materials, strategically placed electron-withdrawing groups may be necessary. |
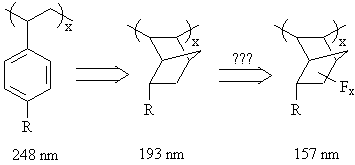 |
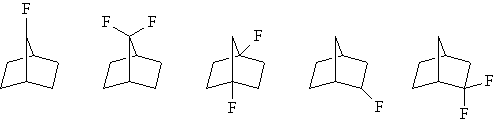
| A series of model norbornene compounds are currently being prepared with varying fluorine substitution patterns to determine the effect on the absorbance at 157 nm. |
 |
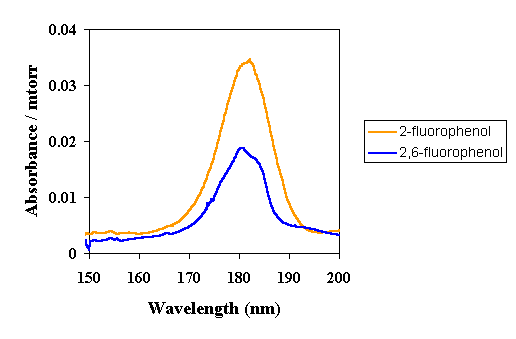
| Our gas phase studies have also shown that the absorbance of aromatic materials can be strongly affected by substituents. By utilizing these effects, it might be possible to use aromatic units in 157 nm photoresists to provide plasma etch resistance. The first series of spectra below show the effect of increased fluorination on phenol. Notice that increased fluorination at the ortho position leads to a decreased absorbance at 157 nm. |
 |
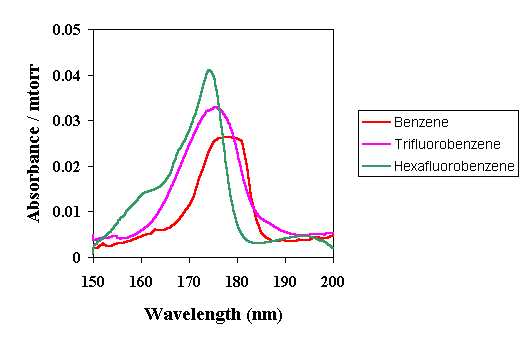
| However, these studies also show that increased fluorination of aromatic materials does not necessarily lead to decreased absorbance values at 157 nm. The seriec of spectra below show the effect of increased fluorination on benzene. In this case, absorbance of these materials at 157 nm actually increases with increased fluorine content. |
 |
| We are in the process of expanding our database of gas-phase absorbance measurements at 157 nm. As this project continues, we expect to develop a better understanding of substituent effects on absorbance at this wavelength. |
| 1) Gokan, H., Esho, S., Ohnishi, Y. J. Electrochem. Soc. 1983, 130, 143. |
