|
|
||
|
Development
of 157 Photoresist Materials
The purpose of this document is to provide timely public access to the 157 nm resist development work being done in our laboratories under contract from International SEMATECH. It is our goal and SEMATECH's goal to disseminate this information as rapidly and widely as possible in hope that doing so will accelerate the availability of 157 nm resist materials to the members of International SEMATECH. Silicon Backbone ModuleEfforts are underway in our research group to synthesize polymers based on siloxane and silsesquioxane structures for 157 nm lithography. The use of these structures holds an advantage over our fluorinated hydrocarbon approach because these Si-O linkages are found to be very transparent at 157 nm. For example, polyhydridosilsesquioxane has an absorbance of 0.01-0.02/um. This polymer is several times lower in absorbance than a typical hydrocarbon polymer at 157 nm, and resists based on this backbone should prove to be transparent as well. SiloxanesPolysiloxanes have the general structure shown in Figure 1. In designing a suitable resist based on this platform, one or both of the R groups must meet several requirements as outlined earlier in our module discussion. To provide etch resistance, R may be an aryl or alicyclic group. It must also be functionalized with an aqueous base-soluble group and be protected with an acid-labile protecting group. Both R groups must not be too absorbing at 157 nm so as to render the entire polymer opaque at that wavelength. 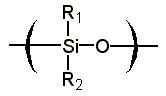 Figure 1. Polysiloxane One other requirement that must be met concerns the glass transition temperature of these siloxane polymers. Siloxanes are known to have very low glass transition temperatures (Table 1) due to the long Si-O bond and O-Si-O bond angle. Therefore, polymers based on this platform must have sufficient Tgs in order to be used as a resist. As seen in the table, the glass transition temperatures of the polymers may be increased by incorporating aromatic or fluorinated groups. Since aromatic groups will render the final polymer to be too opaque at 157 nm, the use of alicyclic groups should provide the same result without increasing absorbance. 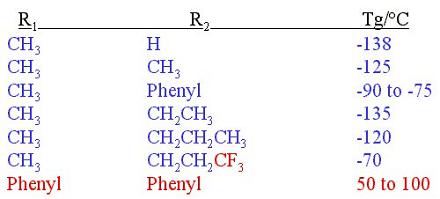 In our group a polysiloxane was made that had an acceptable Tg (Figure 2). This result shows that the use of dinorbornyl groups considerably raises the glass transition temperature of these siloxanes. 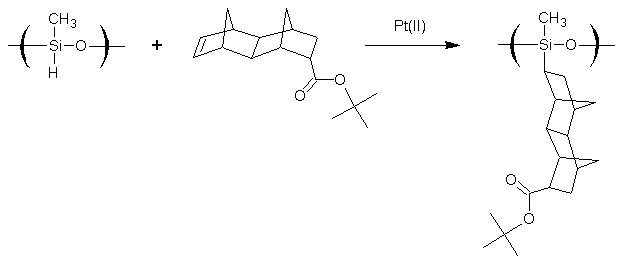 Figure 2. A higher Tg polysiloxane (Tg ~ 90°C) Based on these results, efforts are underway to make polymers based on these structures (Figure 3). 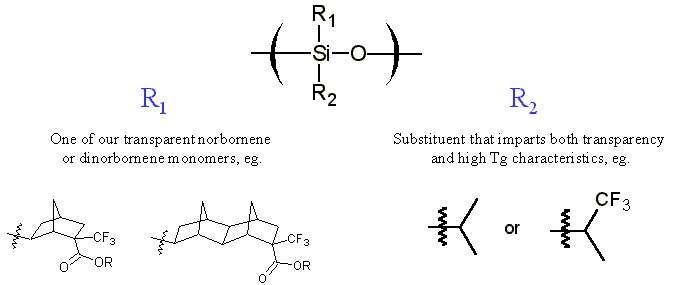 Figure 3. Fluorinated, high Tg polysiloxane SilsesquioxanesA major part of our efforts to make a silicon-containing single-layer positive-tone resist involves the synthesis of functional polysilsesquioxanes. These silsesquioxanes (Figure 4) have some advantages over their siloxane counterparts. They have higher glass transition temperatures because of their crosslinked backbone and are thermally more stable. Because of their crosslinked nature, one would expect that these polymers would not be soluble in most solvents. However, low molecular weight polymers of this structure are soluble in most organic solvents. In bilayer applications, these polymers have another advantage - they are more resistant to oxygen reactive ion etching because their structure is more similar to SiO2. 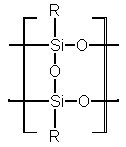 Figure 4. Polysilsesquioxane By hydrosilylating our fluorinated norbornene monomers onto silicon, we are able to make a variety of functionalized silsesquioxane polymers (Figure 5). 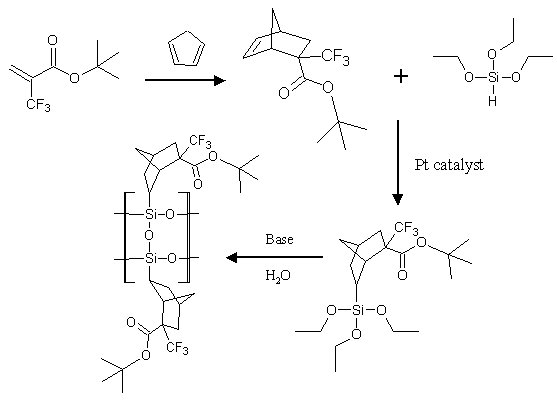 Figure 5. Synthesis of an acid-labile, transparent silsesquioxane Preliminary imaging studies were done with the polymer shown in Figure 5. A positive tone image was obtained from contact printing. Other studies on this polymer, including its absorbance at 157 nm and its coating characteristics, are currently underway. We are currently pursuing and have plans to pursue other polysilsesquioxanes based on the structures shown in the following figures. 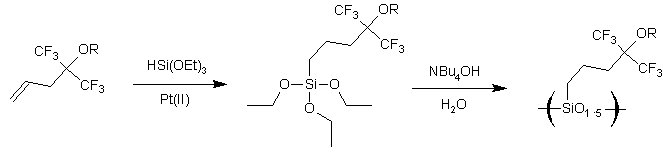 Figure 6. A silsesquioxane from protected allylhexafluoroalcohol 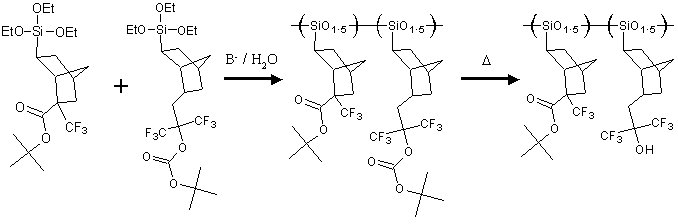 Figure 7. A silsesquioxane copolymer (for better adhesion, higher Tg) 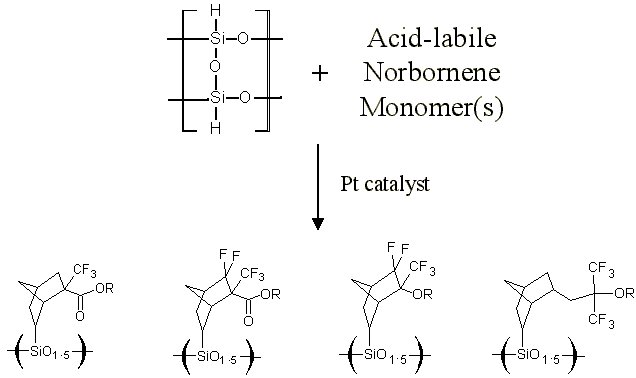 Figure 8. Silsesquioxanes from commercially available hydridosilsesquioxane 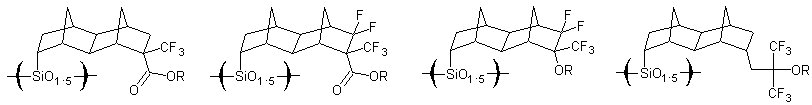 Figure 9. Dinorbornyl silsesquioxanes Version History Original page created 05/28/00 New page created 01/22/01 |
|
|
|
||

