|
|
||
|
Development
of 157 Photoresist Materials
The purpose of this document is to provide timely public access to the 157 nm resist development work being done in our laboratories under contract from International SEMATECH. It is our goal and SEMATECH's goal to disseminate this information as rapidly and widely as possible in hope that doing so will accelerate the availability of 157 nm resist materials to the members of International SEMATECH. Fluorinated Oxetane-Containing Monomers for 157 nm Resist ApplicationsDaniel P. Sanders, Robert H. Grubbs, Brian P. Osborn, Matthew J. Pinnow, Raymond J. Hung, and C. Grant WillsonThe elimination of resist outgassing during exposure and the concomitant fouling of the lithography optics is of increasing importance in each successive generation of lithography. Several groups have shown that fragments of the acid-labile protecting groups account for the vast majority of volatiles produced during resist exposure [1]. Subsequent work towards mass-persistent resists has according focused on protection strategies in which the protecting group is tethered to the polymer. The two predominant structural motifs found in almost every academic and commercial 157 nm photoresist are illustrated in Figure 1 (using the norbornene addition polymer platform as an example). The first is a protected carboxylic acid, usually with an a-trifluoromethyl or other electron-withdrawing group for increased transparency at 157nm. Ongoing research in the Willson laboratory has led to the development of mass-persistent b-lactone structures for 248 nm lithography which upon exposure to a photogenerated acid produce a carboxylic acid without release of any volatiles [2]. The second structural motif is a hexafluorocarbinol functionality in which heavily electron-withdrawing trifluoromethyls lower the pKa of the alcohol such that it resembles the pKa of phenol. Resists for use at 157 nm have been developed based on partial protection of these acidic alcohols, similar to the use of partially-protected PHOST at 248 nm [3]. However, no effort has yet been made develop a mass-persistent version of this resist. 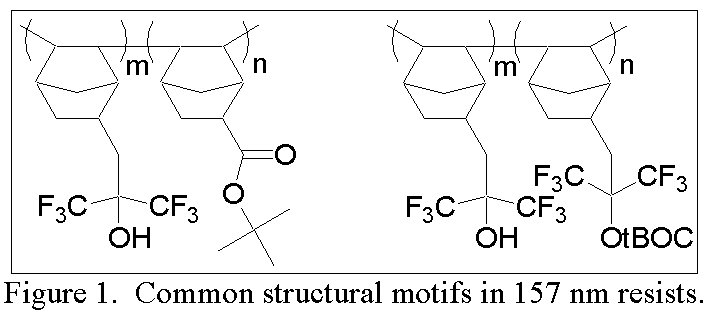
Our collaboration has begun to explore the use of fluorinated oxetane structures for use as transparent and mass-persistent structures capable of forming hexafluorocarbinols upon exposure to photogenerated acids. We have synthesized the tricyclic oxetane-containing monomer 1 for use in 157 nm resist applications. The oxetane ring is attached to the norbornene ring in an exo configuration, bearing a striking resemblance to the tricyclononene structures previously developed by our collaboration for use at 157 nm. 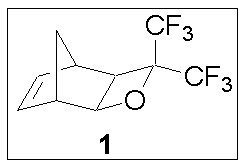
Vacuum UV studies (Chart 1) indicate that the saturated version of this monomer is highly transparent, indicating that this monomer may have broader utility than simply mass-persistent applications. The oxetane ring has been observed to successfully open under acidic conditions to generate hexafluorocarbinol-containing species. 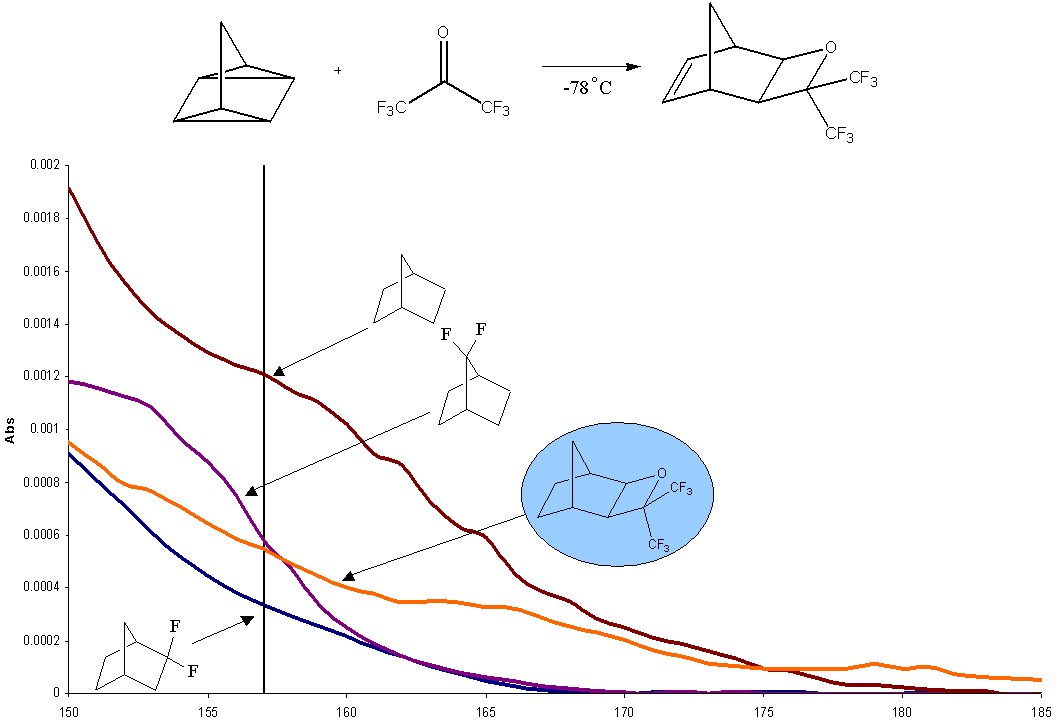
The tricyclic norbornene-like structure serves as a convenient handle though which we aim to polymerize the monomer by metal-catalyzed addition, metal-catalyzed ring-opening metathesis (ROMP), and free radical pathways to make homopolymers and copolymers with various norbornenes or other transparent monomers such as tetrafluoroethylene (Figure 2). 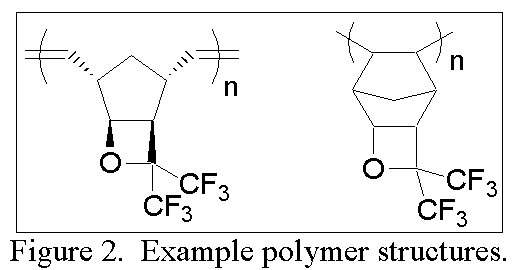
References:
Version History Original page created 01/30/01 Updated 05/21/01 |
|
|
|
||

