|
|
||
|
Development
of 157 Photoresist Materials
The purpose of this document is to provide timely public access to the 157 nm resist development work being done in our laboratories under contract from International SEMATECH. It is our goal and SEMATECH's goal to disseminate this information as rapidly and widely as possible in hope that doing so will accelerate the availability of 157 nm resist materials to the members of International SEMATECH. New Fluorinated Alicyclic Backbone Structures for Vinyl Addition Polymerization and 157 nm PhotoresistsDaniel P. Sanders, Eric F. Connor, Robert H. Grubbs, Raymond J. Hung, Brian P. Osborn, and C. Grant WillsonReplacement of hydrogen with fluorine atoms on norbornene structures has been shown to decrease the absorption of the saturated norbornane model compounds at 157 nm [1]. With this knowledge several fluorinated norbornene (NB) monomers were considered for vinyl addition polymers for 157 nm photoresists, as shown in Figure 1. The hydrogenated model compounds of these monomers showed dramatic reduction in their absorption at 157 nm (compared to non-fluorinated versions). Unfortunately, the geminal substitution of the highly electron-withdrawing trifluoromethyl and ester functionalities at the 2-position of the norbornene produces a highly deactivated norbornene olefin. 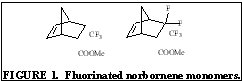 The search for a more reactive olefin led us to consider analogous tricyclononene (TCN) monomers, as shown in Figure 2.  The unfunctionalized TCN alicyclic framework offers a similar Ohnishi parameter (2.33) to norbornene (2.43) and tetracyclododecene (2.33) [2] for high etch resistance [3]. In addition, the synthesis yields only structures with the cyclobutane ring in the exo-configuration [4]. This places the electron-withdrawing functional groups further away from the double bond while leaving the double bond sterically unhindered. Non-fluorinated TCN monomers were shown to be active for both vinyl addition polymerization and free-radical copolymerization with maleic anhydride to produce 193 nm resist-type polymers, as shown in Figure 3. 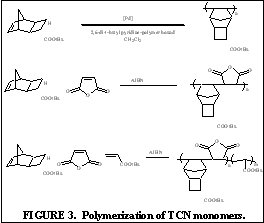 Following this success, fluorinated TCN monomers (I and II) for 157 nm applications were synthesized. The saturated version of II shows high transparency at 157 nm in Figure 4. 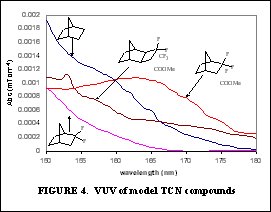 Given these encouraging results, the fluorinated TCN monomers were shown to be active for vinyl addition polymerization with standard palladium catalysts. VASE measurements on the vinyl addition polymer of I show a promising transparency (< 4 mm-1) at 157 nm in Figure 5. 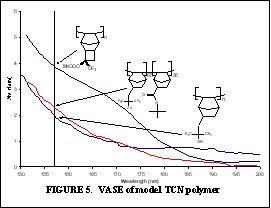 Optical transparency at 157 nm will be increased with the more fluorinated TCN monomer (II) and dilution of the ester by copolymerization with more transparent co-monomers including various hexafluoroisopropyl alcohol functionalized norbornene and TCN monomers. We have shown for the first time the suitability of functionalized-tricyclononene monomers for use as 193 nm and 157 nm photoresists. The wide variety of acrylates, methacrylates, acrylonitriles, methacrylonitriles, anhydrides, and other olefins capable of forming tricyclononene monomers provides for great molecular design flexibility with this backbone structure. Future TCN monomers to be investigated include hexafluoroisopropyl alcohol, trifluoromethyl alcohol, t-butyl ester, and nitrile functionalized TCN monomers among others, some of which are shown in Figure 6. 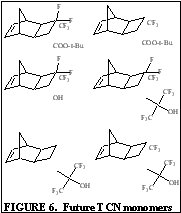 References:
Version History |
|
|
|
||

