|
|
||
|
Development
of 157 Photoresist Materials
The purpose of this document is to provide timely public access to the 157 nm resist development work being done in our laboratories under contract from International SEMATECH. It is our goal and SEMATECH's goal to disseminate this information as rapidly and widely as possible in hope that doing so will accelerate the availability of 157 nm resist materials to the members of International SEMATECH. Condensation Polymers for 157 nm Photoresist MaterialsBrian P. Osborn, Ryan P. Callahan, Shiro Kusumoto, Charles R. Chambers Jr., Scott M. Grayson, Jacob R. Adams and C. Grant WillsonAs previously reported, our attempts to produce a condensation polymer by first making trans-diols of fluorinated norbornane epoxides were initially unsuccessful, instead producing cyclic carbonates and rearranged 2,7-norbornane diols (see original page). We have been able to use the acid-rearranged 2,7-diol structure to our advantage, trapping the dichloroformate of the diol at 0 °C (structure A). Using this dichloroformate and the cis-diol of choice, we were able to produce polycarbonates of the type seen below (Figure 1). 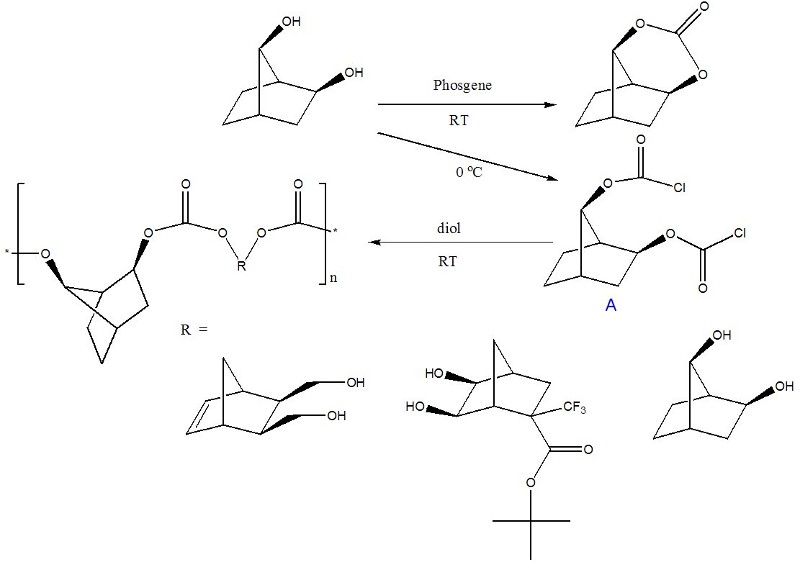
Unfortunately, we were unable to effect similarly high-yielding rearrangements of substituted norbornane epoxides. Therefore, we are currently pursuing alternative means of synthesizing a fluorinated diol (structure B), as seen in Figure 2. We will attempt to produce a condensation polymer in an analogous fashion as in Figure 1 using this structure. 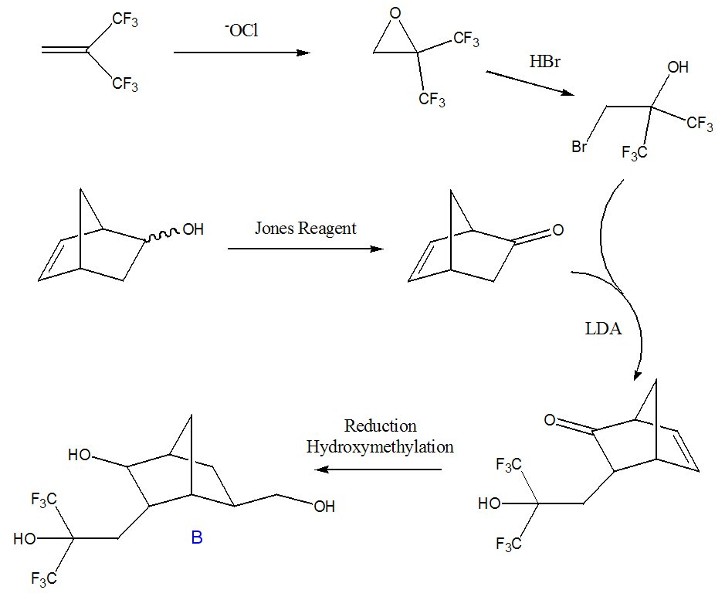
Our conventional efforts to produce a trans-diol from an epoxide were unsuccessful, but we are concurrently pursuing two different synthetic paths to produce the trans-diol of norbornane (Figure 3). The products of both pathways should show identical spectroscopic characterization, with a final X-ray resolved crystallography structure being conclusive proof of the final stereochemistry. 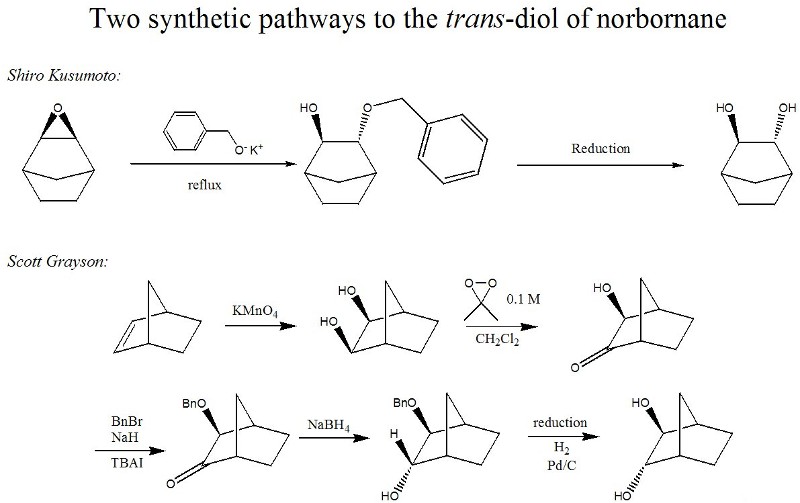
Polyketals are another type of condensation polymer with interesting structures that may be accessible through the synthesis of norbornanes with both vicinal alcohols and a ketone present. This kind of .AB. monomer could self-condense, thus producing a condensation polymer that could include the highly fluorinated and transparent norbornanes we have so far been unable to polymerize by conventional means. Early work in this area with structure C has already produced a condensation polymer (Figure 4). There are other possible monomer structures of the .AB,. .AA. and .BB. type (Figure 4) that can lead to similar polymer structures. 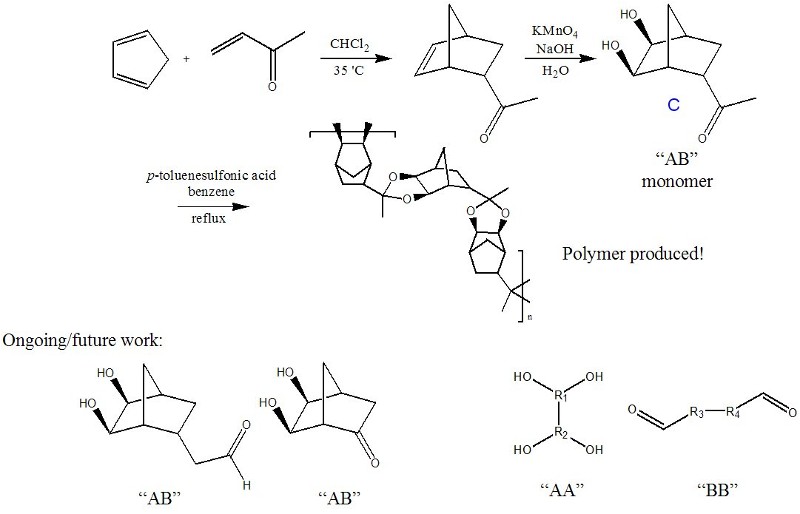
We have also investigated .AB. monomer structures with fluorine incorporationžin this case a hexafluoroisopropyl functional group (structure D). The complete synthetic pathway is shown in Figure 5. We are in the process of scaling up the synthesis, characterization of the polymer and obtaining its absorbance at 157 nm. 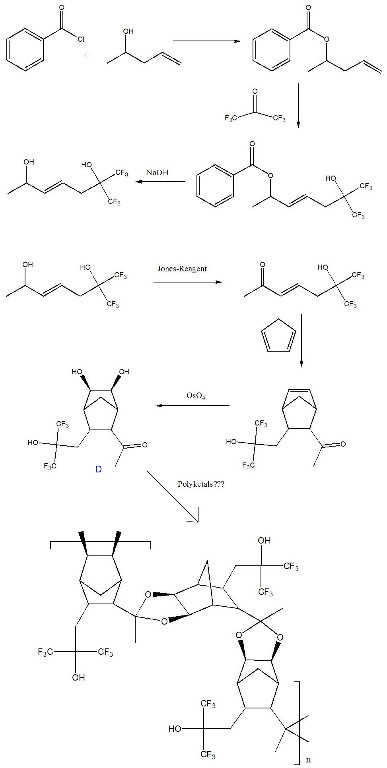
References: Version History Updated page created on 01/13/04 |
|
|
|
||

