|
|
||
|
Development
of 157 Photoresist Materials
The purpose of this document is to provide timely public access to the 157 nm resist development work being done in our laboratories under contract from International SEMATECH. It is our goal and SEMATECH's goal to disseminate this information as rapidly and widely as possible in hope that doing so will accelerate the availability of 157 nm resist materials to the members of International SEMATECH. Condensation Polymers for 157 nm Photoresist MaterialsBrian P. Osborn, Ryan Callahan, Shiro Kusumoto, Charles R. Chambers Jr. and C. Grant WillsonThere are several transparent, highly fluorinated norbornene monomers that have failed to incorporate to any significant extent into an addition or radical polymer. (Figure 1) In an attempt to exploit the transparency of these monomers, we are currently exploring the diol products of olefine oxidization of these norbornenes. Initial V-UV data on these diols shows that these monomers possess a high degree of transparency when compared to the analogous norbornanes. (Figure 2) 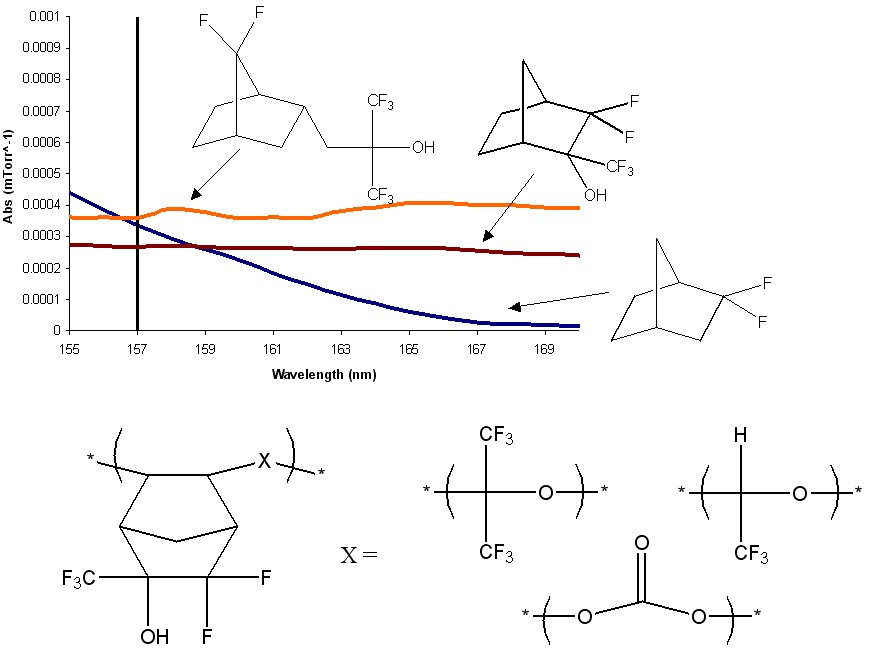
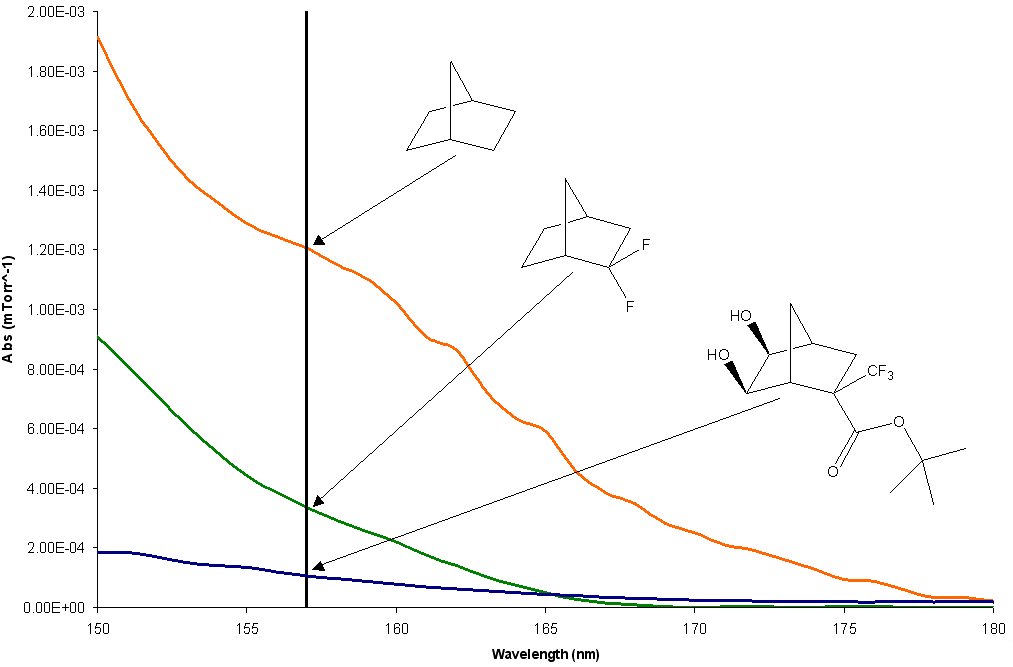
There are several methods by which the diols of norbornanes can be used to create a polymer, including the synthesis of polycarbonates, polyesters and polyethers. (Figure 3) 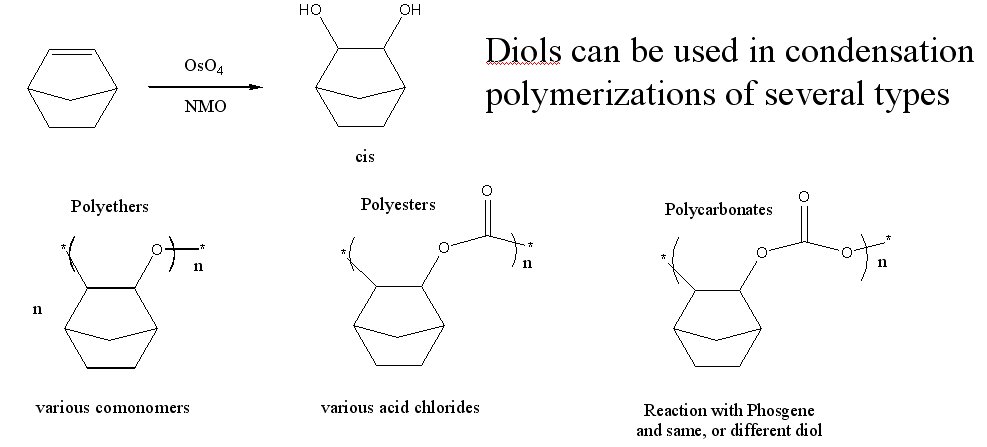
The first attempt to make a condensation polymer was through the use of phosgene, to produce the corresponding polycarbonate. Initial diol synthetic attempts using osmium tetroxide and NMO resulted in the pure cis-diols of norbornane and various substituted norbornanes. Reaction of the norbornane cis-diol with phosgene instead yielding the cyclic carbonate. (Figure 4) All other attempts to modify the reaction and produce polycarbonate only resulted in the cyclic carbonate. 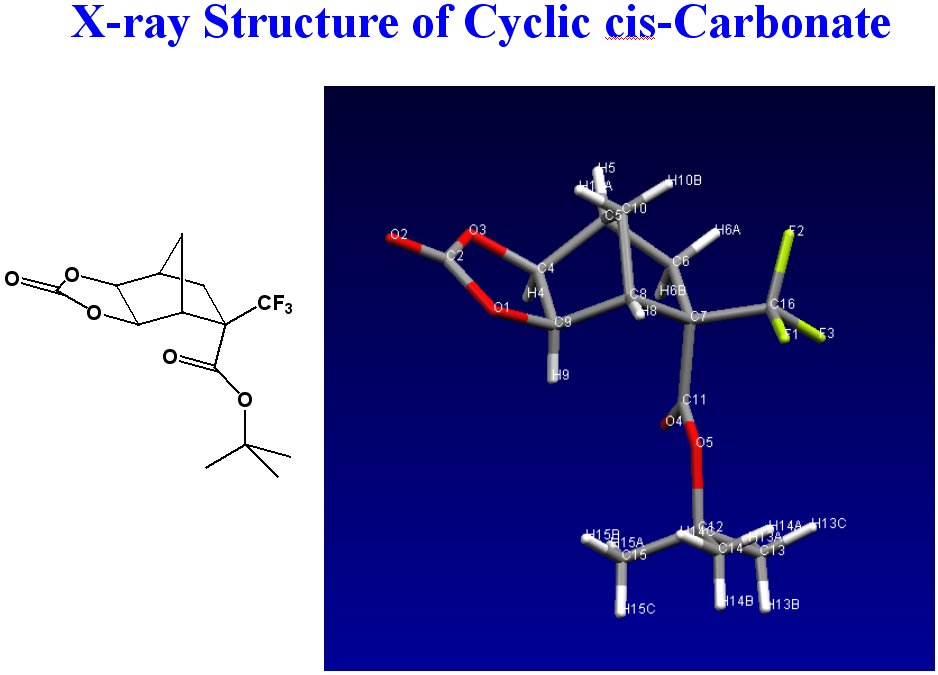
Since work with the cis-diols failed to yield any condensation polymer, our attention turned to forming the trans-diol of our flourinated, transparent norbornanes. There are several possible routes to get to obtain trans-diols from the corresponding epoxides. (Figure 5) 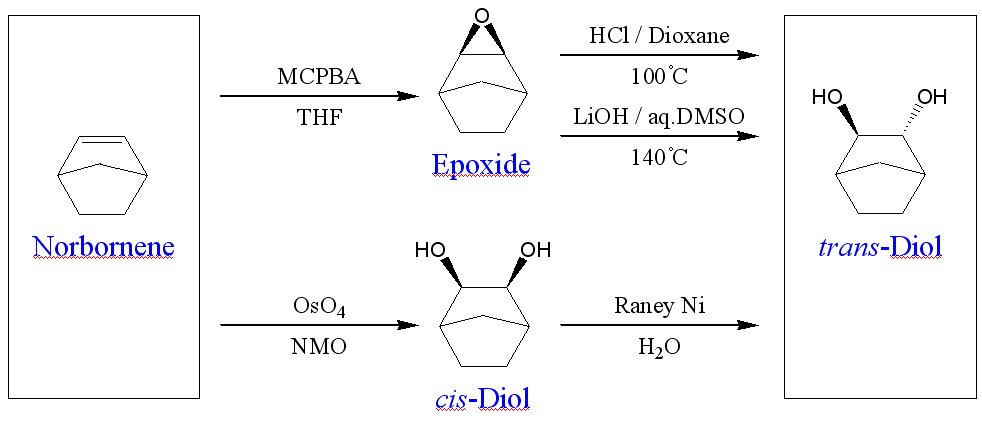
The acidic ring-opening of the exo-2,3-epoxynorbornane did not yield the expected vicinyl diol, but instead yielded the Wagner-Meerwein rearranged 2,7-norbornane diol. This was conclusively determined via X-ray crystallography of the carbamate deriviative of the diol. (Figure 6) We are currently investigating the utility of the base ring-opening epoxide reaction and Raney nickel isomerization of the cis-diol to yield vicinyl norbornane diols. 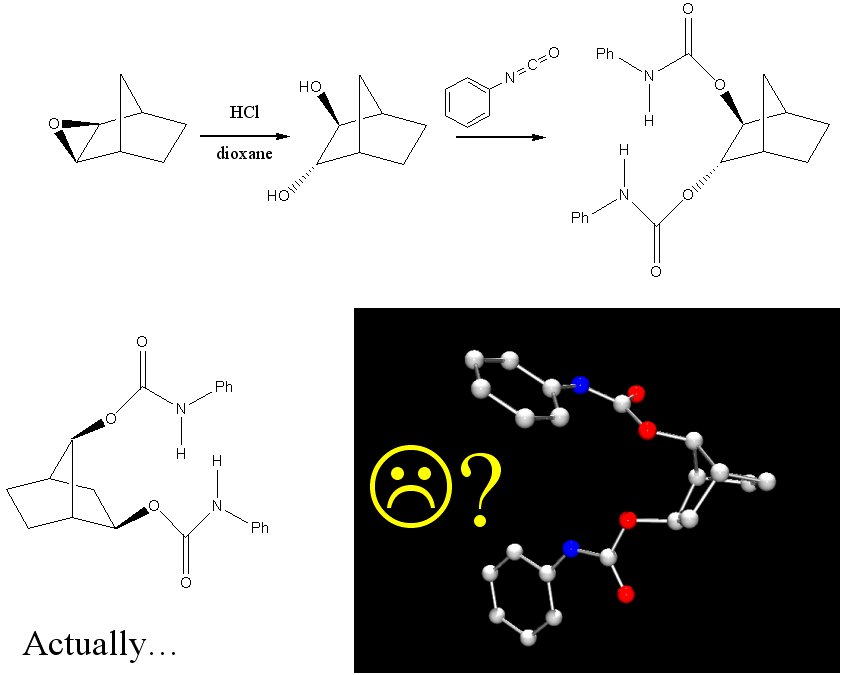
References: Version History Original page created 06/23/03 |
|
|
|
||

