|
|
Development
of 157 Photoresist Materials
The purpose of this document is to
provide timely public access to the 157 nm resist development work being
done in our laboratories under contract from International
SEMATECH. It is our goal and SEMATECH's goal to disseminate this information
as rapidly and widely as possible in hope that doing so will accelerate
the availability of 157 nm resist materials to the members of International
SEMATECH.
New Fluorinated Alicyclic Backbone Structures for Vinyl Addition Polymerization and
157 nm Photoresists
Daniel P. Sanders, Eric F. Connor, Robert H. Grubbs, Raymond J. Hung, Brian P. Osborn, and C. Grant Willson
Replacement of hydrogen with fluorine atoms on norbornene structures has been shown to decrease the absorption of the saturated norbornane model compounds at 157 nm [1]. With this knowledge several fluorinated norbornene (NB) monomers were considered for vinyl addition polymers for 157 nm photoresists, as shown in Figure 1.
 The hydrogenated model compounds of these monomers showed dramatic reduction in their absorption at 157 nm (compared to non-fluorinated versions). Unfortunately, the geminal substitution of the highly electron-withdrawing trifluoromethyl and ester functionalities at the 2-position of the norbornene produces a highly deactivated norbornene olefin.
The hydrogenated model compounds of these monomers showed dramatic reduction in their absorption at 157 nm (compared to non-fluorinated versions). Unfortunately, the geminal substitution of the highly electron-withdrawing trifluoromethyl and ester functionalities at the 2-position of the norbornene produces a highly deactivated norbornene olefin.
The search for a more reactive olefin led us to consider analogous tricyclononene (TCN) monomers, as shown in Figure 2.
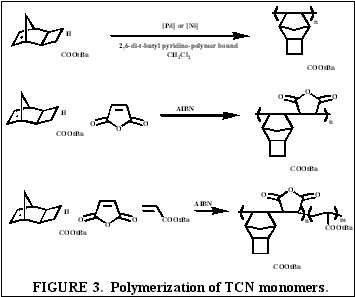
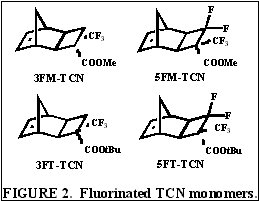 The unfunctionalized TCN alicyclic framework offers a similar Ohnishi parameter (2.33) to norbornene (2.43) and tetracyclododecene (2.33) [2] for high etch resistance [3]. In addition, the synthesis yields only structures with the cyclobutane ring in the exo-configuration [4]. This places the electron-withdrawing functional groups further away from the double bond while leaving the double bond sterically unhindered. Non-fluorinated TCN monomers were shown to be active for both vinyl addition polymerization and free-radical copolymerization with maleic anhydride to produce 193 nm resist-type polymers, as shown in Figure 3.
The unfunctionalized TCN alicyclic framework offers a similar Ohnishi parameter (2.33) to norbornene (2.43) and tetracyclododecene (2.33) [2] for high etch resistance [3]. In addition, the synthesis yields only structures with the cyclobutane ring in the exo-configuration [4]. This places the electron-withdrawing functional groups further away from the double bond while leaving the double bond sterically unhindered. Non-fluorinated TCN monomers were shown to be active for both vinyl addition polymerization and free-radical copolymerization with maleic anhydride to produce 193 nm resist-type polymers, as shown in Figure 3.
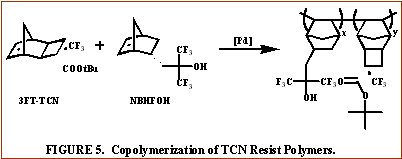
 Following this success, the fluorinated TCN monomers for 157 nm applications shown in Figure 2 were synthesized [5,6]. The saturated version of 5FM-TCN shows high transparency at 157 nm in Figure 4. Given these encouraging results, the fluorinated TCN monomers were shown to be active for vinyl addition polymerization with standard palladium catalysts [5]. VASE measurements on the vinyl addition polymers of 3FM-TCN and 5FM-TCN show promising transparency (< 4 mm-1 and <3 mm-1, respectively) at 157 nm [5,6]. Copolymerization of 3FT-TCN and 5FT-TCN with more transparent co-monomers such as hexafluoroisopropyl alcohol functionalized norbornene (NBHFOH) produces highly transparent, photoresist polymers as shown in Figure 5 [6].
Following this success, the fluorinated TCN monomers for 157 nm applications shown in Figure 2 were synthesized [5,6]. The saturated version of 5FM-TCN shows high transparency at 157 nm in Figure 4. Given these encouraging results, the fluorinated TCN monomers were shown to be active for vinyl addition polymerization with standard palladium catalysts [5]. VASE measurements on the vinyl addition polymers of 3FM-TCN and 5FM-TCN show promising transparency (< 4 mm-1 and <3 mm-1, respectively) at 157 nm [5,6]. Copolymerization of 3FT-TCN and 5FT-TCN with more transparent co-monomers such as hexafluoroisopropyl alcohol functionalized norbornene (NBHFOH) produces highly transparent, photoresist polymers as shown in Figure 5 [6].
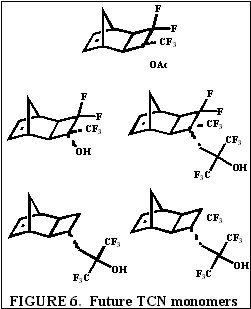 We have shown for the first time the suitability of functionalized-tricyclononene homopolymers and copolymers for use as 193 nm and 157 nm photoresists. The wide variety of fluorinated and non-fluorinated alkene and alkyne compounds including but not limited to acrylates, methacrylates, acrylonitriles, methacrylonitriles, and anhydrides capable of forming tricyclononene monomers will provides for great molecular design flexibility with this backbone structure in photoresist applications. A few representative TCN monomers to be investigated include hexafluoroisopropyl alcohol, trifluoromethyl alcohol, acetate, and nitrile functionalized TCN monomers among others, some of which are shown in Figure 6.
A wide variety of comonomers including but not limited to fluorinated and non-fluorinated acrylates, methacrylates, acrylonitriles, methacrylonitriles, and anhydrides are being evaluated for copolymerization via free-radical and vinyl addition polymerization among others with the various TCN monomers mentioned previously for photoresist applications.
We have shown for the first time the suitability of functionalized-tricyclononene homopolymers and copolymers for use as 193 nm and 157 nm photoresists. The wide variety of fluorinated and non-fluorinated alkene and alkyne compounds including but not limited to acrylates, methacrylates, acrylonitriles, methacrylonitriles, and anhydrides capable of forming tricyclononene monomers will provides for great molecular design flexibility with this backbone structure in photoresist applications. A few representative TCN monomers to be investigated include hexafluoroisopropyl alcohol, trifluoromethyl alcohol, acetate, and nitrile functionalized TCN monomers among others, some of which are shown in Figure 6.
A wide variety of comonomers including but not limited to fluorinated and non-fluorinated acrylates, methacrylates, acrylonitriles, methacrylonitriles, and anhydrides are being evaluated for copolymerization via free-radical and vinyl addition polymerization among others with the various TCN monomers mentioned previously for photoresist applications.
References:
- 1. a). Chiba, T.; Hung, R.J.; Yamada, S.; Trinque, B.; Yamachika, M.; Brodsky, C.; Patterson, K.; Vander Heyden, A.; Jamison, A.; Lin, S-H; Somervell, M.; Byers, J.; Conley, W.; Willson, C.G.; J. Photopolym. Sci. Technol. 2000, 13, 657. b). Brodsky, C.; Byers, J.; Conley, W.; Hung, R.J.; Yamada, S.; Patterson, K.; Somervell, M.; Trinque, B.; Tran, H.V.; Cho, S.; Chiba, T.; Lin, S-H.; Jamison, A.; Johnson, H.; Vander Heyden, T.; Willson, C.G.; J. Vac. Sci. Technol. B. 2000, 18, 3396.
- 2. Douki, K.; Kajita, T.; Shimokawa, T.; Proc. SPIE 2000, 3999(2), 1128.
- 3. Gokan, H.; Esho, S.; Ohnishi, Y.; J. Electrochem. Soc. 1983, 130, 143.
- 4. Tabushi, I.; Yamamura, K.; Yoshida, Z.; JACS 1972, 94, 787.
- 5. Hung, R.J.; Tran, H.V.; Trinque, B.C.; Chiba, T.; Yamada, S.; Sanders, D.P.; Connor, E.F.; Grubbs, R.H.; Klopp, J.; Frechet, J.M.J.; Thomas, B.H.; Shafer, G.J.; DesMarteau, D.D.; Conley, W.; Willson, C.G.; Proc. SPIE, vol. 4345, (in press).
- 6. Sanders, D.P.; Connor, E.F.; Grubbs, R.H.; Hung, R.J.; Osborn, B.P.; Willson, C.G.; 2nd Int. Symp. 157 nm Lithography, Conference held 14-17 May, 2001, Dana Point, CA.
Version History
Original page created 01/30/01
Updated 5/21/01
|



 The hydrogenated model compounds of these monomers showed dramatic reduction in their absorption at 157 nm (compared to non-fluorinated versions). Unfortunately, the geminal substitution of the highly electron-withdrawing trifluoromethyl and ester functionalities at the 2-position of the norbornene produces a highly deactivated norbornene olefin.
The hydrogenated model compounds of these monomers showed dramatic reduction in their absorption at 157 nm (compared to non-fluorinated versions). Unfortunately, the geminal substitution of the highly electron-withdrawing trifluoromethyl and ester functionalities at the 2-position of the norbornene produces a highly deactivated norbornene olefin.
 The unfunctionalized TCN alicyclic framework offers a similar Ohnishi parameter (2.33) to norbornene (2.43) and tetracyclododecene (2.33) [2] for high etch resistance [3]. In addition, the synthesis yields only structures with the cyclobutane ring in the exo-configuration [4]. This places the electron-withdrawing functional groups further away from the double bond while leaving the double bond sterically unhindered. Non-fluorinated TCN monomers were shown to be active for both vinyl addition polymerization and free-radical copolymerization with maleic anhydride to produce 193 nm resist-type polymers, as shown in Figure 3.
The unfunctionalized TCN alicyclic framework offers a similar Ohnishi parameter (2.33) to norbornene (2.43) and tetracyclododecene (2.33) [2] for high etch resistance [3]. In addition, the synthesis yields only structures with the cyclobutane ring in the exo-configuration [4]. This places the electron-withdrawing functional groups further away from the double bond while leaving the double bond sterically unhindered. Non-fluorinated TCN monomers were shown to be active for both vinyl addition polymerization and free-radical copolymerization with maleic anhydride to produce 193 nm resist-type polymers, as shown in Figure 3.
 Following this success, the fluorinated TCN monomers for 157 nm applications shown in Figure 2 were synthesized [5,6]. The saturated version of 5FM-TCN shows high transparency at 157 nm in Figure 4. Given these encouraging results, the fluorinated TCN monomers were shown to be active for vinyl addition polymerization with standard palladium catalysts [5]. VASE measurements on the vinyl addition polymers of 3FM-TCN and 5FM-TCN show promising transparency (< 4 mm-1 and <3 mm-1, respectively) at 157 nm [5,6]. Copolymerization of 3FT-TCN and 5FT-TCN with more transparent co-monomers such as hexafluoroisopropyl alcohol functionalized norbornene (NBHFOH) produces highly transparent, photoresist polymers as shown in Figure 5 [6].
Following this success, the fluorinated TCN monomers for 157 nm applications shown in Figure 2 were synthesized [5,6]. The saturated version of 5FM-TCN shows high transparency at 157 nm in Figure 4. Given these encouraging results, the fluorinated TCN monomers were shown to be active for vinyl addition polymerization with standard palladium catalysts [5]. VASE measurements on the vinyl addition polymers of 3FM-TCN and 5FM-TCN show promising transparency (< 4 mm-1 and <3 mm-1, respectively) at 157 nm [5,6]. Copolymerization of 3FT-TCN and 5FT-TCN with more transparent co-monomers such as hexafluoroisopropyl alcohol functionalized norbornene (NBHFOH) produces highly transparent, photoresist polymers as shown in Figure 5 [6].
 We have shown for the first time the suitability of functionalized-tricyclononene homopolymers and copolymers for use as 193 nm and 157 nm photoresists. The wide variety of fluorinated and non-fluorinated alkene and alkyne compounds including but not limited to acrylates, methacrylates, acrylonitriles, methacrylonitriles, and anhydrides capable of forming tricyclononene monomers will provides for great molecular design flexibility with this backbone structure in photoresist applications. A few representative TCN monomers to be investigated include hexafluoroisopropyl alcohol, trifluoromethyl alcohol, acetate, and nitrile functionalized TCN monomers among others, some of which are shown in Figure 6.
A wide variety of comonomers including but not limited to fluorinated and non-fluorinated acrylates, methacrylates, acrylonitriles, methacrylonitriles, and anhydrides are being evaluated for copolymerization via free-radical and vinyl addition polymerization among others with the various TCN monomers mentioned previously for photoresist applications.
We have shown for the first time the suitability of functionalized-tricyclononene homopolymers and copolymers for use as 193 nm and 157 nm photoresists. The wide variety of fluorinated and non-fluorinated alkene and alkyne compounds including but not limited to acrylates, methacrylates, acrylonitriles, methacrylonitriles, and anhydrides capable of forming tricyclononene monomers will provides for great molecular design flexibility with this backbone structure in photoresist applications. A few representative TCN monomers to be investigated include hexafluoroisopropyl alcohol, trifluoromethyl alcohol, acetate, and nitrile functionalized TCN monomers among others, some of which are shown in Figure 6.
A wide variety of comonomers including but not limited to fluorinated and non-fluorinated acrylates, methacrylates, acrylonitriles, methacrylonitriles, and anhydrides are being evaluated for copolymerization via free-radical and vinyl addition polymerization among others with the various TCN monomers mentioned previously for photoresist applications.