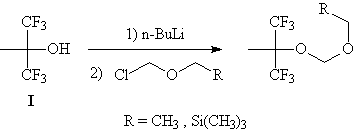| Protecting Group Module |
| To gain an understanding of the appropriate methods for acetal protection of
hexafluoroisopropanol moieties, a series of model studies was carried out using
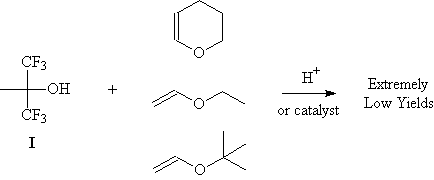
compound I. One common method of acetal formation involves treatment of an alcohol with a vinyl ether
and a catalytic amount of acid (1). Unfortunately, the use of HCl or other acids as the catalyst for this
reaction was completely unsuccessful at preparing the desired acetal. A catalyst reported to aid in the
formation of acetals from tertiary alcohols, triphenylphosphine hydrobromide
(2), was also found to be ineffective in this case. It is possible that a different catalyst
might be capable of mediating this reaction, but we decided to pursue other
methods. |
 |
| Fortunately, there are other pathways for the preparation of
acetals, including the reaction of an alcohol with a chloromethyl ether in the
presence of a base. The treatment of compound I with a strong base such as n-butyllithium or sodium hydride
followed by addition of chloromethyl ethyl ether gave the desired acetal in
encouraging yields. |
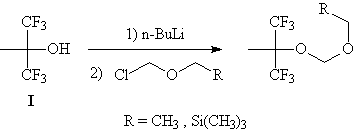 |
| Functional test of hexafluoroisopropanol / acetal combination |
| Although a successful method was found to readily protect
hexafluoroisopropanol functionalities with acetals, it was not clear whether
this combination would be an effective combination in a photoresist. Previous examples of
hexafluoroisopropanols in photoresists consistently utilized tert-butyl
carbonates for the protecting group, and therefore give no indication of what to
expect with acetal protecting groups. As a result, the best method available to test the functional
compatibility of these two moieties is to prepare a model photoresist polymer
and evaluate the resulting lithographic ability. In order to change as few variables as
possible, an existing polymer architecture developed for 193 nm photoresists and
offering well-described lithographic behavior was modified to incorporate an
acetal-protected hexafluoroisopropanol group. |
 |
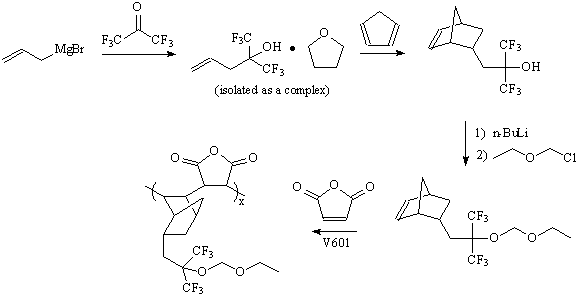
| Synthesis of norbornene monomer:
Synthesis of this copolymer involves the preparation of the
hexafluoroisopropanol-functionalized norbornene, reported by Ito (3). The preparation of this molecule is a
two-step procedure beginning with formation of an allyl hexafluoroisopropanol
imtermediate, through Grignard reaction between allyl magnesium bromide and
hexafluoroacetone (4). The second step involves a Diels-Alder reaction between this intermediate and
cyclopentadiene under elevated heat and pressure. Following the synthesis of the
norbornene monomer and acetal protection of the hydroxyl group with chloromethyl
ethyl ether as outlined for the model compound, the alternating copolymer with
maleic anhydride was prepared using the same procedure outlined for our 193 nm
photoresist materials (5).
|
 |
| A photoresist formulation was prepared from the final copolymer
for lithographic evaluation at an exposure wavelength of 193 nm. As this study was intended only to
verify the compatibility of hexafluoroisopropanol acid groups with acetal
protecting groups, the ultimate resolution capability of this system was
unimportant. Instead, focus was placed on screening the imaging results for phenomena such as undissolved
residue between the patterns (known commonly as scumming) or an unusually high
imaging dose that would indicate an incompatibility between these two functional
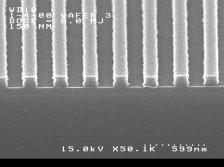
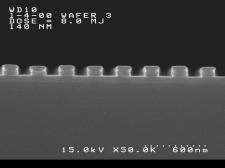
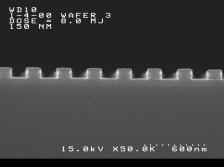
groups. Imaging results are shown below. The
features shown are (left) 140 nm and (right) 150 nm linewidths,
1:1 pitch. The processing conditions were as follows: Film thickness = 125
nm. PAB and PEB = 120ºC / 60s. imaging dose = 8 mJ /cm2, develop 0.26N
TMAH, 30s. |

 |
 |
| FTIR spectra were also taken at various doses prior to development to measure the amount of deprotection.
The figure below shows the normalized concentrations of both the acetal
functional groups (1165-1192 cm-1) and deprotected hydroxyl groups
(3100-3572 cm-1). The experiment was repeated a second time, and both sets of results are shown. |
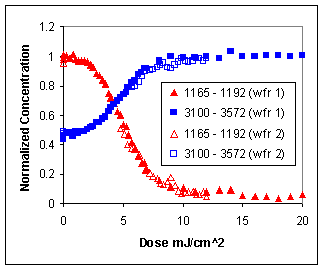 |
| The results from the imaging test are extremely encouraging,
indicating that the deprotection reaction proceeds as expected by FTIR, and no
signs of unfavorable interactions between the acid group and protecting group
are indicated. |
| References |
| 1) Green, T. W., Wuts, P. G. M. “Protective Groups in Organic Synthesis”, John Wiley and Sons,
New York, 1991. |
| 2) Bolitt, V., Mioskowski, C., Shin, D.-S., Falck, J. R. Tetrahedron Letters
1988, 29, 4583. |
| 3) Ito, H., Reichmanis, E., Nalamasu, O., Ueno, T., eds. “Micro- and Nanopatterning
Polymers” American Chemical Society, Washington D.C., 1998, pg 208-223. |
| 4) Okamoto, Y., Yeh, T. F., Lee, H. S., Skotheim, T. A. J. Polym. Sci. Part A: Polym.
Chem. 1993, 31, 2573. |
| 5)Patterson, K., Okoroanyanwu, U., Shimokawa, T., Cho, S., Byers, J. D., Willson, C., G.
Proc. Soc. Photo-Opt. Instrum. Eng. 1998, 3333, 425. |