| Vinyl Addition Polymerization |
| Carbon Monoxide Copolymerization |
| Several transparent disubstituted norbornene monomers discovered
by this lab could not be polymerized with a reasonable yield by conventional
metal catalyzed addition polymerization techniques. Carbon monoxide copolymerization provides one
alternative way to polymerize those transparent geminal disubstituted norbornene monomers (Figure 1). |
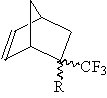 |
| Figure 1. |
| There are two possible backbone structures from carbon monoxide copolymerization;
ketone polymers and spiroketal polymers (1). One advantage of having the ketone moiety in the
backbone is that they can be modified to give a variety of transparent polymer derivatives. (Figure 2) |
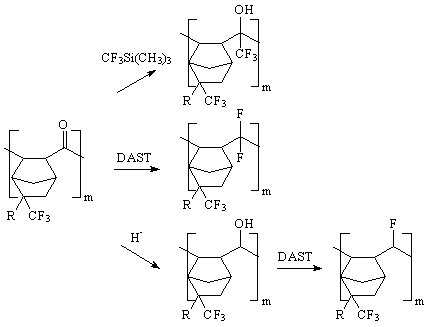 |
| Figure 2. |
| For example, the ketone moiety can be transformed into a trifluoromethyl alcohol group (2)
which not only can reduce the polymerís absorbance at 157 nm, but can also improve the hydrophilicity of the polymer. |
| For the polyketal structure no modification is required if the
monomers used are transparent at 157 nm. We have synthesized some ketal polymers for
VASE measurements (Figures 3 and 4), and are pleased to report that these polymers
show encouraging absorbance at 157 nm. We have also been able to synthesize polymers
2-264 and 2-170 from the transparent geminal disubstituted norbornene monomers.
A carbon monoxide copolymer of SNB-HFOH and SNB-TBE was prepared for preliminary
imaging evaluation at 157 nm (Figure 5). It demonstrated 120 nm lines,
so a carbon monoxide copolymer shows promise. We will synthesize more copolymers
for imaging evaluation and conduct studies to transform the lactone end groups to other
lower absorbing end groups at 157 nm. |
 |
| Figure 3 |
 |
| Figure 4 |
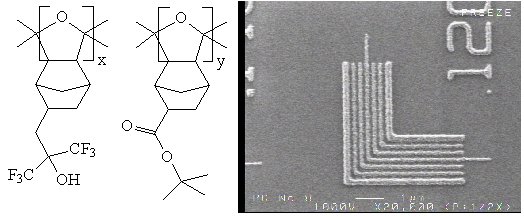 |
| Figure 5 |
- 1. Drent, E.; Budzelaar, P. H. M. Chem. Rev. 1996, 96, 663.
- 2. Prakash, G. K. S.; Krishnamurti, R.; Olah, G. A. J. Amer. Chem. Soc., 1989, 111, 395.
|
| Version History |
|
Original page created 05/28/00
Revised on 1/30/01 |





