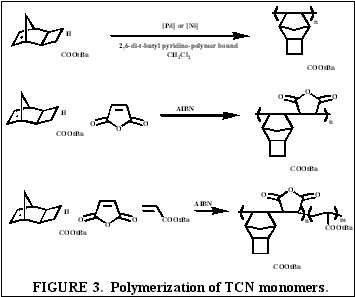
| We have shown for the first time the suitability of functionalized-tricyclononene
homopolymers and copolymers for use as 193 nm and 157 nm photoresists. The wide variety of fluorinated and
non-fluorinated alkene and alkyne compounds including but not limited to acrylates, methacrylates, acrylonitriles,
methacrylonitriles, and anhydrides capable of forming tricyclononene monomers will provides for great molecular design
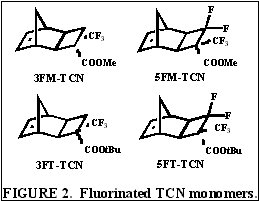
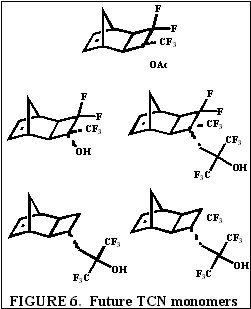
flexibility with this backbone structure in photoresist applications. A few representative TCN monomers to be investigated include
hexafluoroisopropyl alcohol, trifluoromethyl alcohol, acetate, and nitrile functionalized TCN monomers among others, some of which are shown in Figure 6.
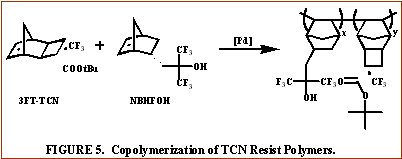
A wide variety of comonomers including but not limited to fluorinated and non-fluorinated acrylates, methacrylates, acrylonitriles, methacrylonitriles,
and anhydrides are being evaluated for copolymerization via free-radical and vinyl addition polymerization among others with the various
TCN monomers mentioned previously for photoresist applications. |
- 1. a). Chiba, T.; Hung, R.J.; Yamada, S.; Trinque, B.; Yamachika, M.; Brodsky, C.; Patterson, K.; Vander Heyden, A.; Jamison, A.; Lin, S-H; Somervell, M.; Byers, J.; Conley, W.; Willson, C.G.; J. Photopolym. Sci. Technol. 2000, 13, 657. b). Brodsky, C.; Byers, J.; Conley, W.; Hung, R.J.; Yamada, S.; Patterson, K.; Somervell, M.; Trinque, B.; Tran, H.V.; Cho, S.; Chiba, T.; Lin, S-H.; Jamison, A.; Johnson, H.; Vander Heyden, T.; Willson, C.G.; J. Vac. Sci. Technol. B. 2000, 18, 3396.
- 2. Douki, K.; Kajita, T.; Shimokawa, T.; Proc. SPIE 2000, 3999(2), 1128.
- 3. Gokan, H.; Esho, S.; Ohnishi, Y.; J. Electrochem. Soc. 1983, 130, 143.
- 4. Tabushi, I.; Yamamura, K.; Yoshida, Z.; JACS 1972, 94, 787.
- 5. Hung, R.J.; Tran, H.V.; Trinque, B.C.; Chiba, T.; Yamada, S.; Sanders, D.P.; Connor, E.F.; Grubbs, R.H.; Klopp, J.; Frechet, J.M.J.; Thomas, B.H.; Shafer, G.J.; DesMarteau, D.D.; Conley, W.; Willson, C.G.; Proc. SPIE, vol. 4345, (in press).
- 6. Sanders, D.P.; Connor, E.F.; Grubbs, R.H.; Hung, R.J.; Osborn, B.P.; Willson, C.G.; 2nd Int. Symp. 157 nm Lithography, Conference held 14-17 May, 2001, Dana Point, CA.
|