| Ring-Opening Metathesis Polymerization (ROMP) of
Functionalized Dinorbornene Monomers for 157 nm Lithography Applications |
| Ring-Opening Metathesis Polymerization |
| ROMP is once again being investigated as a viable polymerization pathway for 157 nm
lithography. In conjunction with Daniel Sanders of
CalTech, ROMP using the Grubbs catalyst and optimal chain transfer agents (CTA) to control polymer
molecular weight. Monomers to be used will be functionalized dinorbornenes in order to increase
the glass transition temperature of the resultant polymers. Primary concern centers on what
trade-off will be paid in optical density with the addition of another norbornene ring. |
| ROMP and 193 nm Lithography |
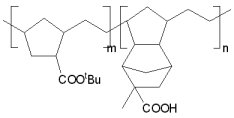 |
| ROMP was previously explored in the Willson group for use in 193 nm lithography, using an
iridium-based catalyst (K2IrCl6). Monomers used were based on norbornenes, with dinorbornenes
used at times as well. During the project, glass transition temperatures were able to be
increased above 130 ºC. However, problems where encountered when trying to image the ROMP
polymers, such as swelling and incompatabilities with some PAGs. |
| ROMP and 157 nm Lithography |
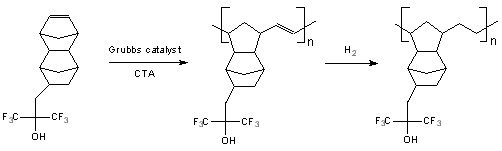
| Now ROMP is being investigated yet again for use at 157 nm, this time using functionalized
dinorbornene monomers to for the first set of polymers. The Grubbs catalyst, a ruthenium-based
catalyst, is being used in lieu of the iridium catalysts. The homopolymer of the dinorbornene
hexafluorocarbinol monomer, which shows that the Grubbs catalyst is tolerant of the highly polar
and acidic hexafluorocarbinol group. |
 |
| Preliminary data shows that the consequence of incorporating a second ring into the monomer
unit is not as high as intuition might suggest. Using Variable Angle Scattering Ellipsometry
(VASE), the homopolymer of the dinorbornene hexafluorocarbinol, cast from cyclohexanone, shows
a thin film absorbance of approximately 3.2/um. These results give reason to further pursue the
ROMP polymers and see if different substituents can yield all the functional modules required for
a polymeric resist material. |
 |
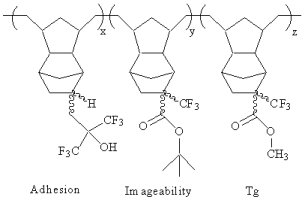
| Future Work |
- Boosting methyl ester content of the polymer to raise the hydrogenated polymerYs Tg
- Copolymerizing with non-functionalized dinorbornene
- Copolymerizing with a variety of functionalized dinorbornene monomers
- Finding optimal chain transfer agents for molecular weight control
|
 |
| References |
- T. Otsuki, K. Goto, Z. Komiya, J. Polym. Sci. A., 2000, 38, 4661.
- C.W. Bielawski, R.H. Grubbs, Angew. Chem. Int. Ed., 2000, 39, 2903.
- M. Scholll, S. Ding, C.W. Lee, R.H. Grubbs, Org. Lett., 1999, 1, 953.
- A.K. Chatterjee, J.P. Morgan, M. Scholl, R.H. Grubbs, JACS, 2000, 122, 3783.
- A.K. Chatterjee, R.H. Grubbs, Org. Lett., 1999, 1, 1751.
|
| Version History |
| Original page created 05/21/01 |




