| Free Radical Polymerization |
| Free radical polymerization of alicyclics |
| A second method of polymer formation with alicyclic monomers is
through copolyermization with electron-poor comonomers such as maleic
anhydride. This was the basis of our research groupís 193 nm photoresist platform(1). Unfortaunely, such materials demonstrate
high absorbance at 157 nm due to both the alicyclic and carbonyl subunits in the repeat unit. However,
we have shown that the absorbance of the alicyclic unit can be attenuated by the addition of fluorine.
We hope to apply the same method to decrease the absorbance of the maleic anhydride comonomer to allow this
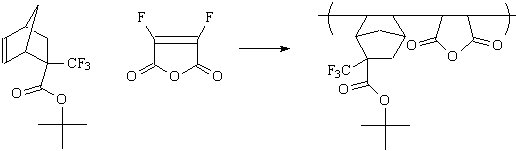
polymer class to be used as 157 nm photoresists. The preparation of difluoromaleic
anhydride has been previously reported(2), and we are currently working to form the promising
copolymer shown below. |
 |
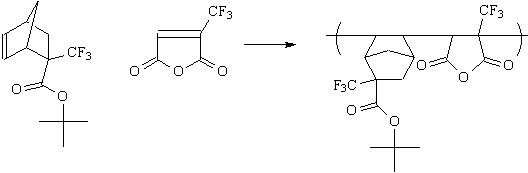
| Another maleic anhydride derivatives we are investigating is
trifluoromethylmaleic anhydride, which is commercially available. We are also working to form the following copolymer. |
 |
| Recently, other electron deficient monomers (other than maleic anhydride derivatives)
have been successfully polymerized with norbornene and substituted norbornenes. The following copolymers have been prepared: |
 |
| All of these monomers are fluorinated or contain a cyano
functional group to impart transparency at 157 nm. They are all commercially available.
In the future we will continue to audition new fluorine or cyano containing monomers.
Vinyl sulfonates will also be investigated as possible radical comonomers. |
|
1)K. Patterson, U. Okoroanyanwu, T. Shimokawa, S. Cho, J.D. Byers, C.G. Willson, "Improving the Performance of 193 nm Photoresists Based on Alicyclic
Polymers", Proc. SPIE, Adv. in Resist Technology and Processing, 1998, 3333, 425. |
|
2)Krespan, U. S. Patent #5,112,993, 1992. |
| Version History |
|
Original page created 05/28/00 |
| Revised on 1/30/01 |
| Revised on 5/21/01 |



