| Acid Group Module |
| Acid Group Module Solubility in industry-standard 0.26 N tetramethylammonium hydroxide (TMAH) solutions is a requirement for all
photoresist polymer designs.Due to the basic nature of TMAH solutions, the solubility requirement is perhaps best
met by designing polymers that offer functional groups of sufficient acidity. For 248 nm photoresists, solubility in TMAH developer solutions is
provided by phenolic moieties that offer a pKa of approximately 10. Unfortunately, absorbance contributions from these aromatic groups
precluded their use in 193 nm photoresist designs, and carboxylic acid derivatives were utilized in their place to provide the necessary acidity. |
|
In order to design appropriate acid groups for 157 nm photoresist polymers, a more fundamental approach must be taken. The stability of the corresponding conjugate base is
responsible for the acidity of a given molecule. In 248 nm photoresist systems, the phenolate conjugate base is stabilized
via resonance involving the adjacent aromatic system. Resonance stabilization is also responsible for the acidity
of carboxylic acids used in 193 nm photoresist designs as the charge of the carboxylate anion is distributed over the two equivalent oxygen atoms.
Unfortunately, studies from MIT Lincoln Labs have shown that materials containing p-systems
will most likely be too absorbing at 157 nm, precluding the use of resonance for conjugate base stabilization. |
|
For 157 nm photoresist designs, inductive stabilization methods will most likely be used instead of the resonance effects utilized in previous
photoresist generations. By placing strongly electron-withdrawing moieties in close proximity, the acidity of a
hydroxyl group can be greatly increased. The negative charge originating from the alkoxide conjugate base is delocalized
through the sigma system of a molecule by the electron-withdrawing groups. Trifluoromethyl groups are ideal electron-withdrawing groups for use in
157 nm photoresist designs as fluorinated materials have been shown to offer decreased absorbance values at this wavelength.
The following table shows that atrifluoromethyl groups can impart a significant increase in acidity through these inductive
effects (1). While typical alcohols present pKa values between 16 and 18, two trifluoromethyl groups
bring this value to approximately 11, similar to that of the phenolic groups utilized for base solubility in 248 nm photoresists. |
|
R1
|
R2
|
pKa
|
|
H
|
H
|
14.5
|
|
H
|
CF3
|
11.2
|
|
CH3
|
CF3
|
11.5
|
|
CF3
|
CF3
|
6.7
|
|
Phenol
|
11.8
|
|
 |
|
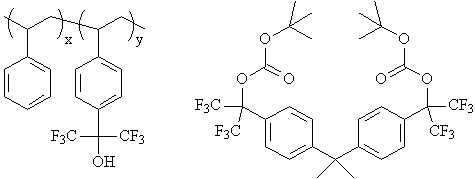
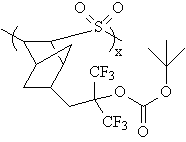
More recently, hexafluoroisopropanol groups were reported in a
193 nm photoresist design (3). In this case, sulfur dioxide was copolymerized with a hexafluoroisopropanol-functionalized
norbornene monomer. As in the previous polystyrene-based photoresist, tert-butyl carbonates were
utilized as acid-labile protecting groups. |
 |
| Absorbance at 157 nm |
|
Although this functionality has been shown by previous examples
to offer sufficient developer solubility, it is critical however that the
overriding transparency requirement for this module not be overlooked.
Unfortunately, the methods available for determining VUV absorbance
values are geared towards film measurements, a technique which does not lend
itself well to this modular approach where the individual absorbance
contributions from small model compounds is of interest.
To alleviate this dilemma, a collaboration was formed with the National
Institute of Standards and Technology (NIST) to make gas-phase VUV absorbance
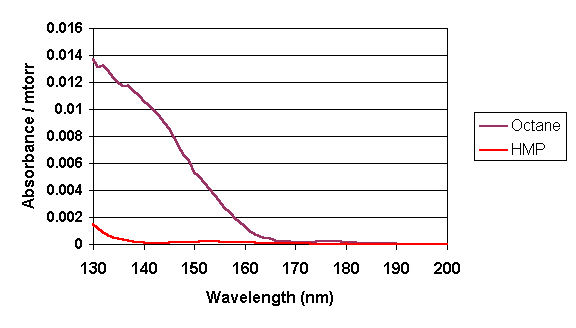
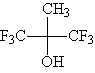
measurements of potential modules candidates. To determine the absorbance contribution of a hexafluoroisopropanol
moiety at 157 nm, the value for 1,1,1,3,3,3-hexafluoro-2-methyl-2-propanol was
measured in the gas phase using the apparatus at NIST.
Relative to simple hydrocarbons such as octane and cyclohexane tested
under the same conditions, this compound is 14-15 times less absorbing at 157 nm
(4). |
 |
|
The model compound:1,1,1,3,3,3-hexafluoro-2-methyl-2-propanol (HMP) |
|
Based on this encouraging absorbance data combined with the
previous successful utilization of hexafluoroisopropanol in photoresist
polymers, it seems likely that the application of these moieties to 157 nm
photoresist polymers will be successful. |
|
1) Grandler,J. R., Jencks, W. P. J. Am. Chem. Soc. 1982, 104, 1937. |
|
2) Przybilla,K. J., Roschert, H., Pawlowski, G. Proc. Soc. Photo-Opt. Instrum. Eng. 1992, 1672, 500. |
|
3) Ito, H., Reichmanis, E., Nalamasu, O., Ueno, T., eds. “Micro- and Nanopatterning Polymers” American Chemical Society, Washington D.C., 1998, pg 208-223. |
|
4) Patterson, K., Yamachika, M., Hung, R., Yamada, S., Brodsky, C., Somervell, M., Osborn, B., Hall, D., Dukovic, G., Byers, J., Conley, W., Willson, C. G. Proc. Soc.
Photo-Opt. Instrum. Eng. 2000, 3999, in publication. |




