|
|
||
|
Aqueous Processable Positive and Negative tone PhotoresistsIntroductionSince the 1980’s, when studies revealed the health effects of exposure to ethylene-based glycol ether solvents, the search for alternative solvent systems has been a consideration of growing significance for resists vendors and semiconductor manufacturers. Propylene-based glycol ethers, lactic acid esters, and a host of other safe solvents have nearly completely supplanted ethylene glycol ethers as photoresist solvents since 1992, when the industry underwent a voluntary phase-out of their use. An additional concern for the semiconductor community is the emission of volatile organic chemical (VOC) vapors. This can become a financial factor as well as an environmental one as some microelectronics fabrication lines can be limited from producing at full capacity due to local restrictions on the amount of VOC’s that may be expelled through their ventilation systems. Furthermore, the growing costs of organic waste treatment and disposal contributes substantially to the cost of operating a microelectronics fabrication line. One approach to limiting the use of organic chemicals in the lithographic imaging processes is to investigate photoresists that can be fully processed with an aqueous media. During the past few years, our research groups have undertaken this approach and have developed a variety of environmentally enhanced resist systems. DesignsWe have developed a variety of photoresist systems in both positive and negative tone in collaboration with Professor Fréchet’s group at UC Berkeley. Our primary goal was to incorporate the means of solubility switching function into the system in order to create resist patterns on a silicon wafer after development. Regarding the negative tone systems, solubility of the film in exposed areas has to be decreased by some photochemical transformations. We have demonstrated this with either a photo-induced cross-linking reaction of poly(MAGME) or a polarity switch via Pinacol rearrangement. Regarding the positive tone, the design of an aqueous positive tone resist is particularly challenging because it requires incorporation of two solubility switches. All the components have to be soluble in water. Thus, the water-soluble formulation must be rendered insoluble by the casting process. We also have to incorporate a mechanism to render the film soluble again on the basis of some photochemical process during exposure. We have managed to achieve the initial insolubilization by either cross-link reaction of vinyl ethers or evaporation of ammonia or a combination of ammonia evaporation and decarboxylation. Negative Tone Resists1) Cross-link system with poly(MAGME) We reported a water castable, water developable negative tone resist based on the acid catalyzed cross-linking of poly(methyl acrylimidoglycolate methyl ether), 1, [poly(MAGME)](1), the components of which are depicted in Scheme 2. The water soluble sulfonium salt 2 was used to selectively produce acids in the exposed areas and 1,4-hexanediol 3 was used as a bifunctional “cross-linker.” Cross-linking results from a variety of mechanisms, including transesterification and alcoholysis of the aminal functionality. Resolution of 1 mm was attainable with this chemically amplified resist system. As can be seen in Figure 1, the resolution of this system was limited by swelling. While cross-linking provided the solubility differential required to define images, a more dramatic contrast in the hydrophilicity between the exposed and unexposed regions is necessary to prevent swelling with water development. 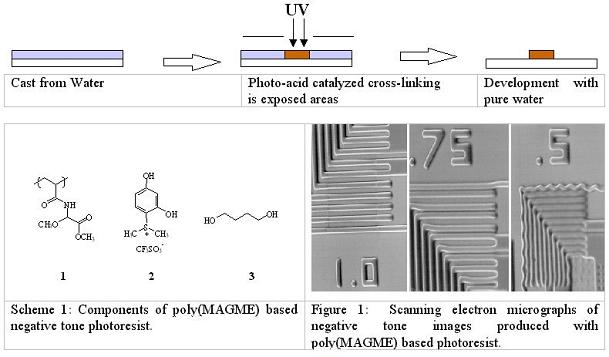
2) Polarity Switch system with Pinacol Rearrangement(2) The concept behind imparting a solubility switch is based on hydrophilic chemical functions in the polymer that can undergo an acid catalyzed transformation resulting in hydrophobic products. Sooriyakumaran et al reported a resist design in which a hydrophilic polymer is converted to a more hydrophobic polymer using Pinacol rearrangement(3). Pinacol rearrangement is an acid catalyzed transformation of vicinal diols into corresponding ketones or aldehydes. Those researchers reported a polymer with pendant vicinal diols that is converted to more lipophilic polymeric ketones upon radiation induced generation of acid. Imaging was accomplished with the diol polymer. However, organic solvent was used as a casting solvent, and methanol was used as a developer because of solubility of the initial and the rearranged polymer. We adopted this chemistry as a switching function. Aqueous solubility of the polymer was increased by incorporating ionic moiety into the polymer as shown in Scheme-2. The copolymer ratio of the polymer 4 was adjusted to be near the threshold in the diol form such that the material is castable from aqueous solution and is rendered insoluble with minimal amount of transformation from the pinacol rearrangement. This system has not demonstrated high resolution imaging because of the availability of good water soluble I-line PAGs. However, the styrenic backbone provided excellent dry etch stability. It was found that this resist system was etched at 67 % the rate of conventional photoresist APEX-Eâ. 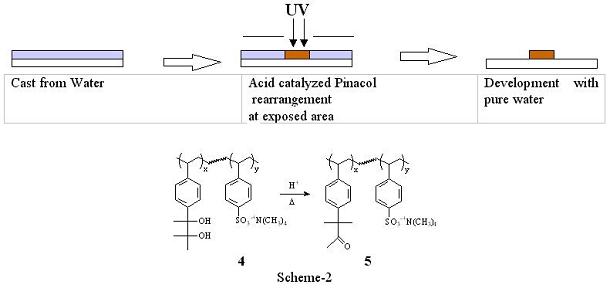
Positive tone1) Cross-link/Decross-link system with vinyl ethers.(2) As previously stated, two solubility switches are necessary to accomplish positive tone imaging in a resist that is both cast from and developed with water. One way to accomplish the two solubility switches is to render the film insoluble by thermally induced cross-linking reaction via acid labile network such as acetals. Upon exposure to UV, photo generated acids cleave the acid labile cross-link site which generates a water-soluble polymer. Yamaoka et al. reported a photoresist system with “two solubility switches” using vinyl ether chemistry.(4) Organic base soluble polymer such as cresol novolak or poly(hydroxystyrene) is thermally insolubilized by the cross-linking reaction via an acetal formation between the acidic hydroxyl group and the vinyl ether moiety. We adopted this vinyl ether system into a water castable, water developable photoresist. We previously reported preliminary evaluation of the cross-linking and decrosslinking of poly(acrylic acid) with a monomeric bisvinyl ether additive in methanol.(5) We since modified this design to include a system in which the vinyl ether functionality is a pendant group on the poly(acrylic acid) backbone, and positive tone imaging that was cast from and developed with water was accomplished. Since carboxylic acids readily add to vinyl ethers in aqueous solution, the reactive carboxylic acids were “protected” as their salts. The ammonium salt copolymer, 6, is highly water soluble and casts high quality films. Upon PAB, ammonia was volatilized from the films and the corresponding free carboxylic acid was formed. The free acid quickly reacted with the vinyl ether groups to form acetals. This reaction cross-linked the film, 7, and rendered it aqueous insoluble, as is represented in Scheme 3. Photogenerated acid and water hydrolyze the acetals in the irradiated areas. The acid catalyzed “de-crosslinking” regenerated aqueous developable material, 8, and a gaseous by-product, acetaldehyde. The basic design has been verified and we have demonstrated positive tone images in a fully water processable photoresist. However, this positive tone resist has a variety of shortcomings. As expected, the plasma and reactive ion etch resistance of these materials is poor. A thin film of polymer 7, for example, etched at a rate over three times that of the commercial resist APEX-E®. The photoresist formulated with polymer 6 gave no images after 6 days even though the sample was stored in the refrigerator at 8 ºC. This indicates that the system is subject to a slow “dark reaction” and therefore have limited shelf life. 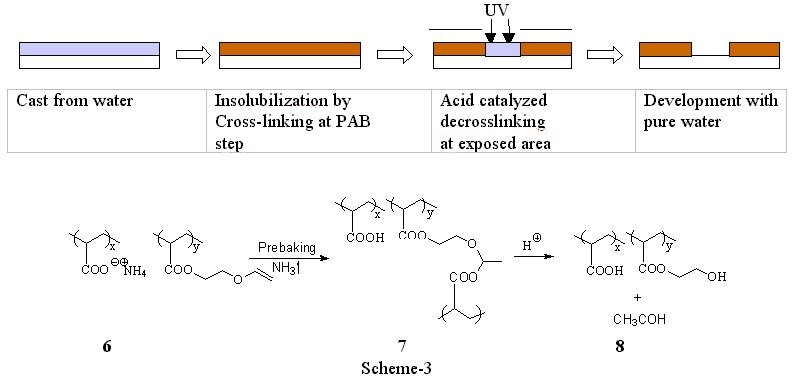
2) Polarity switch system with evaporation of ammonia Havard et al proposed an insolubilization mechanism using volatile amines(5). For example, ammonia was added to a water insoluble acidic polymer. The acid forms an ammonium salt which increases aqueous solubility of the polymer. Upon PAB, ammonia is evaporated such that the polymer returns to original insoluble form. Once the polymer film is insolubilized, photoacid-catalyzed thermolysis, the reaction commonly used in chemically amplified resists, renders exposed areas soluble in aqueous base developer providing positive tone images. 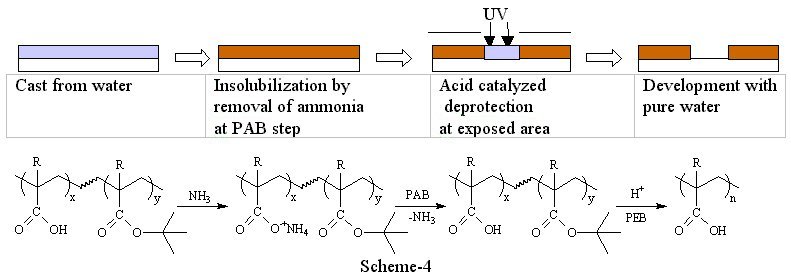
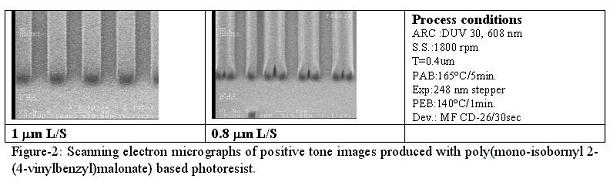
3) Decarboxylation system(6) The use of volatile ammonia was proved to be an effective way to increase the solubility of polymers in water. However, this method makes polymers too soluble when conventional TMAH developer was required to use. There are still some demands to use TMAH developer in industry because the development lines have been well established. Since TMAH is a stronger base than ammonia, the polymer is soluble in TMAH if the initial polymer is soluble in aqueous ammonia. Therefore, we need one more trick to render the film insoluble in aqueous base after casting. Scheme-6 shows an effective way to incorporate the double switching function into a photo-polymer that we recently explored. The styrenic polymer backbone was chosen for this design in order to provide sufficient dry etch stability. The ammonium salts of the half esters of malonic acids provide not only the initial solubility of polymer but also the insolubilization mechanism at post application baking via sequential volatilization of ammonia and decarboxylation. Once the film is insolubilized, photoacid-catalyzed thermolysis, the reaction commonly used in chemically amplified resists, renders exposed areas soluble in aqueous base developer providing positive tone images. The thermal stability of the acid labile protecting group is critical in order to obtain the double switching function. For example, tert-butyl ester decomposes during the decarboxylation process. Therefore, it resulted in poor imaging contrast. Isobornyl ester is more thermally stable than tert-butyl ester, and still cleavable with photo-acids. Polymers bearing isobornyl ester showed an excellent reaction selectivity between the decarboxylation and the thermal decomposition of the ester. Preliminary imaging of this system showed 1 mm resolution with 248 nm exposure and standard TMAH developer (Figure-2). Further modification of polymer structure, formulation, process conditions and detailed kinetic studies are in progress. 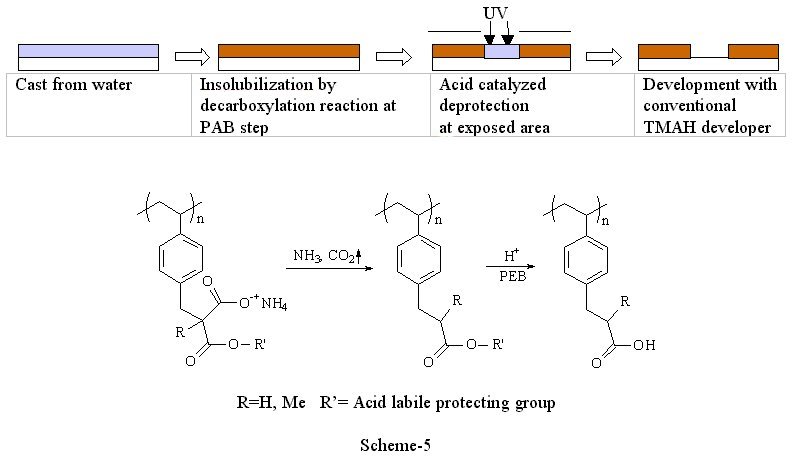
References
Version History Original page created 04/18/01
| |
|
|
||

