Introduction |
Background |
| Everyday experience has led to universal familiarity with the three states of matter: solids, liquids, and gases. However, there are many organic materials that demonstrate more than a single transition in passing from a liquid to a solid, thereby neces sitating the description of one or more intermediate phases. The mechanical properties and intermolecular packing arrangements of these phases are in between those of a liquid and those of a crystal. They have partial ordering of molecules similar to so lids, while maintaining the ability to flow akin to liquids. Therefore, these materials are classified as liquid crystals and exhibit one or more liquid crystalline phases, or more properly, mesophases. |
| The study of liquid crystals began in 1888 when Friedrich Reinitzer[1], an Austrian botanist, observed that a material known as cholesteryl benzoate had two distinct melting points. Since then, it has been shown that numerous compounds exhibit this type of behavior. However, it was not until the last twenty years that this area of materials research experienced explosive growth, primarily due to the successful applications of liquid crystals in the area of electro-optical displays. |
| There are two main classifications of liquid crystals, thermotropic and lyotropic, which are distinguished by the mechanisms that drive their self-organization. Lyotropic liquid crystal transitions occur with the influence of solvents and these mesogens are of great interest biologically and appear to play an important role in living systems. However, thermotropic liquid crystals are the focus of this research and are characterized by mesophase transitions which are most naturally effected by changing t emperature. A particular class of thermotropic liquid crystals are made up of molecules with a rod-like structure. These molecules typically possess rigidness of the long axis with strong dipoles and/or easily polarizable substituents. The distinguishi ng characteristic of these rod-like molecules is the tendency of the molecules to point along a common axis called the director. A further classification is drawn from the relative extent of lattice order preserved in the mesophase. For example, in the isotropic state, there is no order of the director relative to other molecules. The nematic phase demonstrates one-dimensional orientational order, i.e., the molecules tend to align parallel to each other; however, no positional order is present. A further degree of order is present in the smectics. Not only do the molecules align with parallel directors, but they also arrange in regularly spaced layers. It should be noted that these phases are temperature dependent and a single liquid crystall ine material can exhibit multiple mesophases. In the smectic A phase, the director is perpendicular to the layer while the molecules in the smectic C phase are uniformly tilted with respect to the layer normal. |
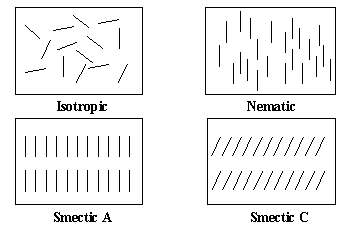 |
| Figure 1: Molecular ordering of various thermotropic liquid crystal phases. Note: As the order of a phase increases, the temperature at which these phases are found decreases. |
| Molecules which exhibit a Smectic C phase contain a polar substituent resulting in a dipole moment orthogonal to the long axis of the molecule. In order to minimize intermolecular repulsions combined with sterical considerations, the molecular axis tilt with respect to the layer normal. Therefore, this phase possesses the following monoclinic symmetry elements: a two-fold axis of rotation perpendicular to the long molecular axes, a mirror plane parallel to the layers, and a center of inversion. Howeve r, if a chiral center is introduced in the molecule, then the mirror plane and the center of inversion are eliminated, leaving only the polar C2 axis of rotation[2]. |
| A molecule of this type was theoretically predicted by R.B. Meyer et. al.[3] to be ferroelectric. For a material to be ferroelectric, the polar axis must be along a unique rotation axis to which no perpendicular plane of reflection symmetry belong s[4]. A chiral Smectic C (SmC*) is ferroelectric due to the inequivalence of the dipoles along the C2 axis, even though the molecules are undergoing rapid reorientational motion about their long axis[5]. This time dependent alignment of dipoles along th e C2 axis causes a spontaneous polarization to arise parallel to the smectic layer planes, resulting in a spontaneous polarization for each individual layer. To ensure a zero bulk polarization, the spontaneous polarization from layer to layer is s hifted slightly resulting in a helielectric phase[6]. Unwinding of this helix results in the layer polarizations all aligned in the same direction and the entire bulk phase becomes ferroelectric. |
| Clark and Lagerwall[7] were the first to take advantage of the ferroelectric nature of the SmC* phase to produce an electro-optical device, known as the surface stabilized ferroelectric liquid crystal display (SSFLCD). This device requires unwinding of t he helical structure to ensure common alignment of the electric dipoles. This is accomplished using glass cells spaced closer than the ferroelectric helix pitch (the length required for one complete revolution of the helix). The cell surfaces are coate d with a polyimide and unidirectionally rubbed, causing the molecular layers near the surface to align with the direction of rubbing. This results in smectic layers arranged perpendicular to the glass plates and is known as the "bookshelf" arrangement. |
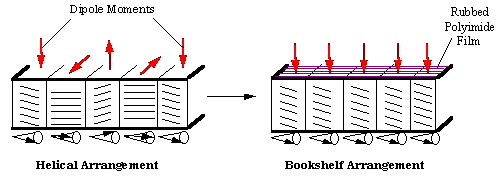 |
| Figure 2: Helical arrangement of the SmC* phase versus the bookshelf arrangement. Dark black lines represent the boundaries of the glass cells, vertical lines represent smectic layers, and the parallel lines within the cell represent ind ividual molecules. The cones show the precession of molecular axes at constant azimuthal angle about the layer normal, i.e., one complete rotation about the cone represents the helical pitch. |
| There is a net polarization resulting from the aligning of the dipoles which is also perpendicular to the glass plates. This polarization can couple to an externally applied field to rotate the molecules either "up" or "down" resulting in either of two energetically degenerate states, or bistability. |
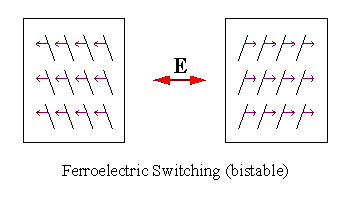 |
| Figure 3: Bistable switching of a ferroelectric liquid crystal in a bookshelf geometry. Note: Small arrows represent polarization direction and E is the electric field. |
| Since these molecules are optically anisotropic in nature, the amount of polarized light transmitted through such a material is strongly dependent on the molecular orientation. Therefore, ferroelectric liquid crystals can act as optical shutters by the a pplication of an alternating field when viewed between crossed polarizers. Unfortunately, the interaction of the molecules with the rubbed polyimide surface is too weak to ensure a durable cell. Surface perturbations can cause the loss of the bookshelf arrangement, whereby the molecules immediately revert to their helical conformation. Moreover, this molecular reordering is irreversible and causes loss of function of the display. |
| In 1988, Chandani et. al.[8] reported the existence of another tilted smectic phase named the antiferroelectric phase and designated it as SmCA* In an antiferroelectric ordering, molecules reverse their tilt directions on passing from layer to lay er, where the polarization vectors in subsequent layers point in opposite directions, thereby canceling each other[9]. As a consequence, the spontaneous polarization falls to zero and the driving force for a helical arrangement is removed. One can think of the antiferroelectric ordering in terms of a ferroelectric helix where the pitch length has been reduced to two adjacent smectic layers. This phase shows tristable switching as shown in Figure 4 below. |
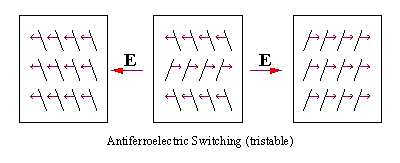 |
| Figure 4: Three stable electric states of the antiferroelectric phase with small arrows representing polarization vectors and E is the electric field. |
| Antiferroelectric materials have also been used for electro-optical devices. They have the advantage of no net spontaneous polarization in the absence of an electric field, meaning they orient strongly with the surface since there is no helical structure to unwind. A positive or negative field of sufficient strength causes the rotation of molecular dipoles in alternating layers such that all the directors now align. Thus, a ferroelectric bookshelf geometry results, which will again have a natural tende ncy to form a helix upon loss of the surface stabilization. However, unlike the SSFLCD, this surface stablilization can be regained by simply removing the field, resulting in the return of the antiferroelectric phase which aligns well with the surface. |
| Liquid crystal polymers (LCP) represent another active area of research for use in advanced display devices. LCP incorporate a unique combination of characteristics peculiar to liquid crystals, i.e., polarizability, self assembly and fast response times to external stimuli, and those typical to macromolecules, such as durability, ease of processability and dimensional stability[10]. Also, doping of LCP with a low molar mass ferroelectric liquid crystal of a similar design allows for more competit ive switching speeds in conjunction with the added durability gained from the stabilizing matrix of the LCP. |
| Another attempt to gain a partial stability advantage over singular molecular systems is to link together two mesogenic units to form dimeric or twin liquid crystals. These YSiamese-twin mesogensY [11] consist of two identical mesogenic units which have been linked in one of a variety of ways. These include; fused twins, such as those studied by Kelker et. al. [12] and Malthete et.al. [13] where the mesogenic units are linked rigidly by a ring system; the well studied head to head twins[14 -16] which consist of the mesogenic units linked together at their ends; or ligated twins where the molecules are connected by a short spacer in a central position as first presented by Griffin et. al. [17] and continued by Weissflog [18,19]. |
| Project Aim: Flat panel display technology constitutes the fastest growing segment of the semiconductor industry. These displays take advantage of the fact that many of the physical properties of liquid crystalline materials, such as birefringence, viscosity, and the rmal conductivity are sensitive to relatively weak external stimuli, including electric fields, magnetic fields, heat energy, and acoustical energy. Presently, the majority of display related research is focused on the application of electro-optic effect s due to the ease and efficiency of molecular reorientation with an applied voltage as compared with other means of stimulation. One such area of study is the surface stabilized ferroelectric liquid crystal (SSFLC) display, which has many advantages over conventional cathode ray tubes as well as other types of liquid crystal displays. The inherent limitation encountered in SSFLC displays is that the interaction between liquid crystal molecules and the device surfaces is too delicate to provide true surf ace stabilization, which inevitably leads to device failure. It is the goal of this research to overcome this shortcoming by designing liquid crystal molecules which will have strong surface stabilization, yet maintain the desirable switching characteris tics found in traditional ferroelectric liquid crystals. In order to accomplish this, these molecules are designed to have the advantages of dimeric, oligomeric, or polymeric stability while incorporating the tristable states found in antiferroelectric m esogens. |
| References |
| 1. F. Reinitzer, Monatsh Chem., 1888, 9, 421. |
| 2. J.W. Goodby, Ferroelectrics, 1983, 49, 275. |
| 3. R.B. Meyer, L. Liebert, L. Strzelecki, P. Keller, Le Journal De Physique Letters, 1975, 36, L69. |
| 4. S.T. Lagerwall and I. Dahl, Mol. Cryst. Liq. Cryst., 1984, 114, 151. |
| 5. J.W. Goodby, J. Mater. Chem., 1991, 1, 307. |
| 6. H.R. Brand, P.E. Cladis, P.L. Finn, Phys. Rev. A, 1985, 31, 361. |
| 7. N.A. Clark and S.T. Lagerwall, Appl. Phys. Lett., 1980, 36, 899. |
| 8. A.D.L. Chandani and H. Takezoe, Jpn. J. Appl. Phys., 1988, 27, 729. |
| 9. I. Nishiyama, Adv. Mater., 1994, 6, 966. |
| 10. E. Chiellini, G. Galli, F. Cioni, E. Dossi, Makromol. Chem. , Macromol. Symp., 1993, 69, 51. |
| 11. J. Malthete, J. Billard, J. Jacues, C. R. Acad. Sc. Paris, 1975, 281, 333. |
| 12. H. Kelker and B. Scheurle, Molec. Crystals, 1969, 7, 381. |
| 13. J. Malthete, C. R. Acad. Sc. Paris, 1983, 296, 435. |
| 14. C. Aguilera, S. Ahmad, J. Bartulin, H. Muller, Mol. Cryst. Liq. Cryst., 1988, 162B, 277. |
| 15. A. Ferrarini, G.R. Luckhurst, P.L. Nordio, S.J. Roskilly, Chem. Phys. Letters, 1993, 214, 409. |
| 16. A.C. Griffin, S.R. Vaidya, R.S.L. Hung, S. Gorman, Mol. Cryst. Liq. Cryst. Letters, 1985, 1, 131. |
| 17. A.C. Griffin, S.F. Thames, M.S. Bonner, Mol. Cryst. Liq. Cryst. Letters, 1977, 34, 135. |
| 18. H. Dehne, A. Roger, D. Demus, S. Diele, H. Kresse, G. Pelzl, W. Wedler, W. Weissflog, Liquid Crystals, 1989, 6, 47. |
| 19. W. Weissflog, D. Demus, S. Diele, P. Nitschke, W. Wedler, Liquid Crystals, 1989, 5, 111. |
