|
|
||
|
Development
of 157 Photoresist Materials
The purpose of this document is to provide timely public access to the 157 nm resist development work being done in our laboratories under contract from International SEMATECH. It is our goal and SEMATECH's goal to disseminate this information as rapidly and widely as possible in hope that doing so will accelerate the availability of 157 nm resist materials to the members of International SEMATECH. Multi-functional Monomers for 193 nm and 157 nm PhotoresistsDaniel P. Sanders, Prof. Robert H. Grubbs – California Institute of TechnologyBrian P. Osborn, Prof. C. Grant Willson – Univ. of Texas – Austin Addition polymers of ester-functionalized norbornenes tend to exhibit swelling during development. This swelling can be alleviated via the use of various dissolution inhibitors. However, another possible approach is to incorporate a second functionality into the monomer which can serve as a hydrogen bond donor/acceptor and moderate the hydrophilicity of the carboxylic acid moiety. 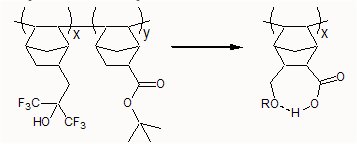
Amongst the large number of possible hydrogen-bonding functionalities, a hexafluorocarbinol group is an ideal candidate for this second functionality for use in 193 nm and 157 nm lithography. While hexafluorocarbinol-functionalized norbornenes have become ubiquitous amongst the majority of 157 nm resist platforms under development due to their excellent transparency and hydrophilicity, ester-functionalized norbornenes are simply not transparent enough to use at 157 nm unless a trifluoromethyl group is incorporated alpha to the ester. Unfortunately, this substitution pattern renders the norbornene inactive towards polymerization by standard metal addition catalysts and necessitates the use of alternative alicyclic frameworks such as tricyclononenes (TCN) in order to utilize these transparent esters. 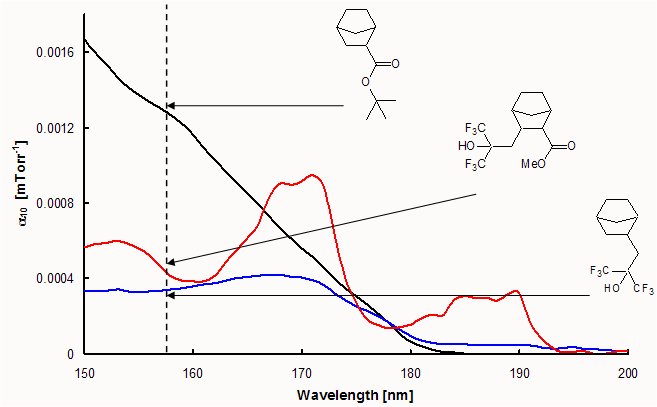
To examine whether the hexafluorocarbinol group would impart sufficient transparency to the monomer, a model compound was synthesized and examined via vacuum UV spectroscopy. Surprisingly, the addition of the hexafluorocarbinol imparts considerable transparency to the monomer. This result opens up a possible facile route to the inclusion of transparent ester-functionalized norbornenes and norbornene-like structures into metal-catalyzed addition and ring-opening metathesis polymers as well as free radical polymers, including copolymers with tetrafluoroethylene, to provide resist materials with modified dissolution behavior. A wide range of multi-functional norbornene and tricyclononene monomers (a few are illustrated below) are being explored for potential use as positive or negative tone resists for use in deep UV lithography. 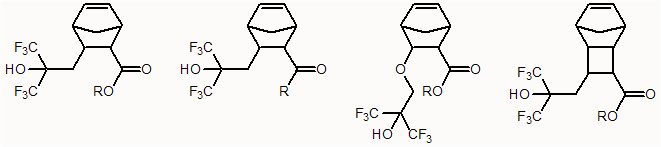
Version History Page created on 03/03/04 |
|
|
|
||

